Abstract
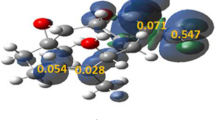
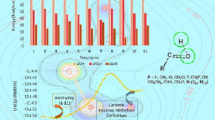
In this work, a systematic study on the kinetics and mechanism of ring-opening reaction of oxirane by carboxylic acid initiated by a tertiary amine is presented. Kinetic parameters of β-hydroxypropyl ester formation including reaction orders, rate constants, and activation energies were established at the temperature range 323–353 K. The experimental values of Δ≠H° and Δ≠S° are characteristic for the SN2-like processes. In the initial reaction system, the acid, oxirane and amine exist mainly as hydrogen-bonded complex acid-oxirane, free oxirane and free base. H-bonding was analyzed using IR-spectroscopy. The reaction pathways were examined by the density functional theory (DFT) method at the B3LYP/6-31+G** level. Optimized equilibrium configurations of transition states and corresponding activation parameters were established. In accordance to both experimental and theoretical approaches, it is reasonable to suggest that amine-catalyzed ring-opening reaction of oxirane by carboxylic acid is a series of parallel consecutive stages: (1) quaternization of tertiary amine by activated oxirane; (2) carboxylate anion participation in ring-opening of both nonactivated and activated oxirane. The kinetic model, which adequately describing all observations, is proposed.














Similar content being viewed by others
References
Wicks ZW, Jones FN, Pappas SP (2007) Organic coatings science and technology, 3rd edn. Wiley, Hoboken
Gilbert M (ed) (2016) Brydson’s plastics materials, 8th edn. Butterworth-Heinemann, Oxford
Meninno S, Lattanzi S (2016) Chem Eur J 22:3632–3642
Choi BS, Choi J, Bak S, Koo S (2015) Eur J Org Chem 3:514–524
Blank WJ, He ZA, Picci M (2002) J Coat Technol 74:33–41
Sinel’nikova MA, Shved EN (2014) Russ J Org Chem 50:332–336
Kakiuchi H, Tanaka Y (1966) J Org Chem 31:1559–1564
Tanaka Y (1967) J Org Chem 32:2405
Mares F, Hetflejs J, Bazant V (1969) Collect Czech Chem Commun 34:3086–3097
Hetflejs J, Mares F, Bazant V (1969) Collect Czech Chem Commun 34:3098–3109
Shvets VF, Romashkin AV (1972) Kinet Katal 13:885–891
Ricci CG, Cabrera MI, Luna JA, Grau RJ (2002) Synlett 11:1811–1814
Brønsted JN, Pedersen KJ (1924) Stöchiometrie und verwandtschaftslehre. Z Phys Chem 108:185–235
Swain CG, Scott CB (1953) J Am Chem Soc 75:141–147
Bakhtin S, Bespal’ko Y, Shved E (2016) Reac Kinet Mech Cat 119:139–148
Cheng G-J, Zhang X, Chung LW, Xu L, Wu Y-D (2015) J Am Chem Soc 137:1706–1725
Ly UQ, Pham M-P, Marks MJ, Truong TN (2017) J Comput Chem 38:1093–1102
Bakhtin S, Shved E, Bespal’ko Y (2017) J Phys Org Chem 30:3717–3726
Nikol’skii BP (ed) (1971) Spravochnik khimika (chemist’s handbook). Khimiya, Moscow
Perrin DD, Amarego WLF (1988) Purification of laboratory chemicals. Pergamon Press, Oxford
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347–1363
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Becke AD (1993) J Chem Phys 93:5648–5652
Parr RG, Yang W (1989) Density-functional theory of atoms and molecules. Oxford University Press, New York
Scott AP, Radom L (1996) J Phys Chem 100:16502–16513
Bouteiller Y, Gillet J-C, Gregoire G, Schermann JP (2008) J Phys Chem A 112:11656–11660
Gonzales C, Schlegel HB (1989) J Chem Phys 90:2154–2161
McQuarrie DA (2000) Statistical Mechanics. University Science Books, Sausalito
Takao I (1979) Tetrahedron 35:299
Derevyanko LI (1972) Ukr Khim Zh 38:771
Gordon AJ, Ford RA (1972) The chemist’s companion. Wiley, New York
Okovytyy S (2014) In: Gorb L, Kuzmin V, Muratov E (eds) Application of computational techniques in pharmacy and medicine, challenges and advances in computational chemistry and physics. Springer, Dordrecht
Acknowledgements
Funding was provided by Ministry of Education and Science of Ukraine (Grant No. 0116U002519).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bespalko, Y.N., Shved, E.N. Experimental and theoretical study on the kinetics and mechanism of the amine-catalyzed reaction of oxiranes with carboxylic acids. Reac Kinet Mech Cat 126, 903–919 (2019). https://doi.org/10.1007/s11144-018-01524-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-018-01524-2