Abstract
Background
Previous reports on daptomycin’s adverse drug reactions (ADRs) have been insufficient, often because of limited data. Pharmacovigilance risk signal detection is innovative and has been applied to the safety monitoring and reevaluation of drugs post-marketing.
Aim
The study aimed to promote safe daptomycin prescribing by mining and evaluating the daptomycin ADR signals from the US Food and Drug Administration Adverse Event Reporting System (FAERS).
Method
A disproportionality analysis (reporting odds ratio ROR and proportional reporting ratio PRR) was utilized for FAERS data mining from the first quarter of 2004 to the second quarter of 2021 (the most recent quarterly data at the time of the study). Preferred Terms of ADR reports were categorized by System Organ Class (SOC) based on the Medical Dictionary for Regulatory Activities.
Results
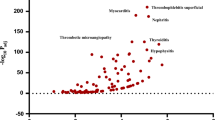
This study retrieved 12,221 cases within the reporting period. A total of 140 repetitive signals were obtained by ROR and PRR, of which 53 new ADR signals were not recorded in the drug labels/datasheets. The top three ADR reports were “blood creatine phosphokinase elevation” (ROR, 56.66, 95% confidence interval (CI) 51.07–62.87, PRR 51.94), “eosinophilic pneumonia” (ROR 696.71, 95%CI 603.21–804.70, PRR 657.57), and “rhabdomyolysis” (ROR 22.85, 95%CI 19.94–26.18, PRR 21.83). The highest ROR of “antimicrobial susceptibility test resistant” was found at 9808.14. Reports of rare adverse events, such as “necrotizing fasciitis and compartment syndrome,” have emerged. The significant SOCs were “Infections and Infestations” and “Investigations.”
Conclusion
New daptomycin ADR signals were detected. Clinicians should monitor these potential ADRs in patients receiving daptomycin.

Similar content being viewed by others
References
Heidary M, Khosravi AD, Khoshnood S, et al. Daptomycin. J Antimicrob Chemother. 2018;73(1):1–11.
Humphries RM, Pollett S, Sakoulas G. A current perspective on daptomycin for the clinical microbiologist. Clin Microbiol Rev. 2013;26(4):759–80.
Taylor SD, Palmer M. The action mechanism of daptomycin. Bioorg Med Chem. 2016;24(24):6253–68.
Kirkpatrick P, Raja A, LaBonte J, et al. Daptomycin. Nat Rev Drug Discov. 2003;2(12):943–4.
Fowler VG Jr, Boucher HW, Corey GR, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355(7):653–65.
Sader HS, Farrell DJ, Flamm RK, et al. Daptomycin activity tested against 164457 bacterial isolates from hospitalised patients: summary of 8 years of a Worldwide Surveillance Programme (2005–2012). Int J Antimicrob Agents. 2014;43(5):465–9.
Pertel PE, Bernardo P, Fogarty C, et al. Effects of prior effective therapy on the efficacy of daptomycin and ceftriaxone for the treatment of community-acquired pneumonia. Clin Infect Dis. 2008;46(8):1142–51.
Ye Y, Xia Z, Zhang D, et al. Multifunctional Pharmaceutical Effects of the Antibiotic Daptomycin. Biomed Res Int. 2019;20198609218.
Abraham G, Finkelberg D, Spooner LM. Daptomycin-induced acute renal and hepatic toxicity without rhabdomyolysis. Ann Pharmacother. 2008;42(5):719–21.
Bohm N, Makowski C, Machado M, et al. Case report and cohort analysis of drug-induced liver injury associated with daptomycin. Antimicrob Agents Chemother. 2014;58(8):4902–3.
Arrieta AC, Bradley JS, Popejoy MW, et al. Randomized multicenter study comparing safety and efficacy of daptomycin versus standard-of-care in pediatric patients with staphylococcal bacteremia. Pediatr Infect Dis J. 2018;37(9):893–900.
Shrestha NK, Mason P, Gordon SM, et al. Adverse events, healthcare interventions and healthcare utilization during home infusion therapy with daptomycin and vancomycin: a propensity score-matched cohort study. J Antimicrob Chemother. 2014;69(5):1407–15.
Rege S, Mohr J, Lamp KC, et al. Safety of daptomycin in patients completing more than 14 days of therapy: results from the cubicin ® Outcomes Registry and experience. Int J Antimicrob Agents. 2013;41(5):421–5.
He W, Zhang Y, Chen H, et al. Efficacy and safety of daptomycin for the treatment of infectious disease: a meta-analysis based on randomized controlled trials. J Antimicrob Chemother. 2014;69(12):3181–9.
Kim PW, Sorbello AF, Wassel RT, et al. Eosinophilic pneumonia in patients treated with daptomycin: review of the literature and US FDA adverse event reporting system reports. Drug Saf. 2012;35(6):447–57.
Mo Y, Nehring F, Jung AH, et al. Possible hepatotoxicity associated with daptomycin: a case report and literature review. J Pharm Pract. 2016;29(3):253–6.
Britt NS, Potter EM, Patel N, et al. Comparison of the effectiveness and safety of linezolid and daptomycin in vancomycin-resistant enterococcal bloodstream infection: a national cohort study of veterans affairs patients. Clin Infect Dis. 2015;61(6):871–8.
Schäublin M. Pharmacovigiance: the spontaneous reporting system in Switzerland. Ther Umsch. 2015;72(11–12):743–8.
Yamada T, Watanabe Y, Kusama M, et al. Factors associated with spontaneous reporting of adverse drug reactions in Japan. Pharmacoepidemiol Drug Saf. 2013;22(5):468–76.
Shin YS, Lee YW, Choi YH, et al. Spontaneous reporting of adverse drug events by Korean regional pharmacovigilance centers. Pharmacoepidemiol Drug Saf. 2009;18(10):910–5.
Sakaeda T, Tamon A, Kadoyama K, et al. Data mining of the public version of the FDA adverse event reporting system. Int J Med Sci. 2013;10(7):796–803.
Gatti M, Fusaroli M, Raschi E, et al. Serious adverse events with tedizolid and linezolid: pharmacovigilance insights through the FDA adverse event reporting system. Expert Opin Drug Saf. 2021;20(11):1421–31.
Peng L, Xiao K, Ottaviani S, et al. A real-world disproportionality analysis of FDA adverse event reporting system (FAERS) events for baricitinib. Expert Opin Drug Saf. 2020;19(11):1505–11.
Subeesh V, Maheswari E, Singh H, et al. Novel adverse events of iloperidone: a disproportionality analysis in US food and drug administration adverse event reporting system (FAERS) database. Curr Drug Saf. 2019;14(1):21–6.
Lertxundi U, Erezuma I, Hernandez R, et al. Antipsychotics and pituitary tumors: an analysis of the European pharmacovigilance database (EudraVigilance). Int Clin Psychopharmacol. 2019;34(2):89–92.
Neha R, Beulah E, Anusha B, et al. Aromatase inhibitors associated osteonecrosis of jaw: signal refining to identify pseudo safety signals. Int J Clin Pharm. 2020;42(2):721–7.
Sakaeda T, Kadoyama K, Minami K, et al. Commonality of drug-associated adverse events detected by 4 commonly used data mining algorithms. Int J Med Sci. 2014;11(5):461–5.
Xu R, Wang Q. Large-scale combining signals from both biomedical literature and the FDA Adverse Event Reporting System (FAERS) to improve post-marketing drug safety signal detection. BMC Bioinformatics. 2014;1517.
Wilson AM, Thabane L, Holbrook A. Application of data mining techniques in pharmacovigilance. Br J Clin Pharmacol. 2004;57(2):127–34.
Hauben M, Zhou X. Quantitative methods in pharmacovigilance: focus on signal detection. Drug Saf. 2003;26(3):159–86.
Ooba N, Kubota K. Selected control events and reporting odds ratio in signal detection methodology. Pharmacoepidemiol Drug Saf. 2010;19(11):1159–65.
van Puijenbroek EP, Bate A, Leufkens HG, et al. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 2002;11(1):3–10.
Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 2001;10(6):483–6.
Long X, Gan X, Zeng X. Signal detection and evaluation of adverse reactions induced by regorafenib. Cent South Pharm. 2019;17(10):1798–802.
Konychev A, Heep M, Moritz RK, et al. Safety and efficacy of daptomycin as first-line treatment for complicated skin and soft tissue infections in elderly patients: an open-label, multicentre, randomized phase IIIb trial. Drugs Aging. 2013;30(10):829–36.
Bland CM, Bookstaver PB, Lu ZK, et al. Musculoskeletal safety outcomes of patients receiving daptomycin with HMG-CoA reductase inhibitors. Antimicrob Agents Chemother. 2014;58(10):5726–31.
Oleson FB Jr, Berman CL, Kirkpatrick JB, et al. Once-daily dosing in dogs optimizes daptomycin safety. Antimicrob Agents Chemother. 2000;44(11):2948–53.
Dvorchik BH, Brazier D, DeBruin MF, et al. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob Agents Chemother. 2003;47(4):1318–23.
Wu D, Xing Y, Zhen J. Literature analysis of rhabdomyolysis induced by daptomycin. Adverse Drug React. 2017;19(04):272–6.
Chuma M, Nakamoto A, Bando T, et al. Association between statin use and daptomycin-related musculoskeletal adverse events: A mixed approach combining a meta-analysis and a disproportionality analysis. Clin Infect Dis. 2022.
Suzuki Y, Suda T. Eosinophilic pneumonia: a review of the previous literature, causes, diagnosis, and management. Allergol Int. 2019;68(4):413–9.
Hagiya H, Hasegawa K, Asano K, et al. Myopathy and eosinophilic pneumonia coincidentally induced by treatment with daptomycin. Intern Med. 2015;54(5):525–9.
Silverman JA, Mortin LI, Vanpraagh AD, et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis. 2005;191(12):2149–52.
Arbeit RD, Maki D, Tally FP, et al. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin Infect Dis. 2004;38(12):1673–81.
Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med. 2002;136(11):834–44.
Kołpa M, Wałaszek M, Gniadek A, et al. Incidence, Microbiological Profile and Risk Factors of Healthcare-Associated Infections in Intensive Care Units: A 10 Year Observation in a Provincial Hospital in Southern Poland. Int J Environ Res Public Health. 2018;15(1).
Kulldorff M, Dashevsky I, Avery TR, et al. Drug safety data mining with a tree-based scan statistic. Pharmacoepidemiol Drug Saf. 2013;22(5):517–23.
Acknowledgements
None
Funding
This study was supported by the Shandong Pharmaceutical Association Special Scientific Research and Academic Activity Project (Grant number yyyx2021qn-13). The title of the project is "clinical study on the safety evaluation of daptomycin and its effect on CPK based on the FAERS database".
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, JJ., Huo, XC., Wang, SX. et al. Data mining for adverse drug reaction signals of daptomycin based on real-world data: a disproportionality analysis of the US Food and Drug Administration adverse event reporting system. Int J Clin Pharm 44, 1351–1360 (2022). https://doi.org/10.1007/s11096-022-01472-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-022-01472-x