Abstract
Background Bone metastases-induced skeletal complications result in reduced patient survival, lower quality of life, and an increase in healthcare costs. Previously, zoledronic acid (ZA) was the standard choice of treatment for bone metastases, but another drug, denosumab, has also shown promise. However, the clinical utility of these two drugs requires further exploration. Aim of the review Due to the lack of direct comparisons regarding the efficacy of these drugs in both solid tumors and multiple myeloma (MM), we herein tried to conduct a meta-analysis to compare their efficacy in parallel for bone metastases treatment in both solid tumor and MM patients. Methods Multiple databases including Cochrane Library, MEDLINE, EMBASE, and Web of Science were searched to identify randomized controlled trials (RCTs) reported up to March 2019 directly comparing denosumab with ZA in solid tumors and MM. Information about the following events was primarily searched: time to first on-study skeletal-related event (SRE), time to first and subsequent SREs, and overall survival. Information about secondary outcomes including disease progression, pain, health-related quality of life, and adverse events was also recorded. Results Overall, we analyzed data from four distinct RCTs including 7441 patients, and our analysis revealed that patients in the denosumab group had a significantly delayed incidence to the first and subsequent SREs. In addition, denosumab resulted in a higher incidence of hypocalcemia and osteonecrosis of the jaw (ONJ), and a lower incidence of renal toxicity and acute phase reactions, in comparison to ZA. Conclusion Overall, denosumab showed superiority in delaying the first and subsequent SREs, and hence seems to be a promising choice for managing bone metastases in both solid tumors and MM. However, it can induce a higher incidence of ONJ and hypocalcaemia, but these are preventable and manageable effects.





Similar content being viewed by others
References
Kruger TE, Miller AH, Godwin AK, Wang J. Bone sialoprotein and osteopontin in bone metastasis of osteotropic cancers. Crit Rev Oncol Hematol. 2014;89:330–41.
Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–9s.
Macedo F, Ladeira K, Pinho F, Saraiva N, Bonito N, Pinto L, Goncalves F, et al. Bone metastases: an overview. Oncol Rev. 2017;11:321.
Costa L, Badia X, Chow E, Lipton A, Wardley A. Impact of skeletal complications on patients’ quality of life, mobility, and functional independence. Support Care Cancer. 2008;16:879–89.
Leppert W. Pain management in patients with cancer: focus on opioid analgesics. Curr Pain Headache Rep. 2011;15:271–9.
Schulman KL, Kohles J. Economic burden of metastatic bone disease in the U.S. Cancer. 2007;109:2334–42.
Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J, E.G.W. Group. Bone health in cancer patients: ESMO clinical practice guidelines. Ann Oncol. 2014;25(Suppl 3):124–37.
Rosen LS, Gordon D, Tchekmedyian NS, Yanagihara R, Hirsh V, Krzakowski M, et al. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, Phase III, double-blind, placebo-controlled trial. Cancer. 2004;100:2613–21.
Grávalos C, Rodríguez C, Sabino A, Seguí MÁ, Virizuela JA, Carmona A, et al. SEOM clinical guideline for bone metastases from solid tumours (2016). Clin Transl Oncol. 2016;18:1243–53.
Terpos E, Kleber M, Engelhardt M, Zweegman S, Gay F, Kastritis E, et al. European Myeloma Network guidelines for the management of multiple myeloma-related complications. Haematologica. 2015;100:1254–66.
Smith MR. Zoledronic acid to prevent skeletal complications in cancer: corroborating the evidence. Cancer Treat Rev. 2005;31(Suppl 3):19–25.
McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354:821–31.
Yee AJ, Raje NS. Denosumab for the treatment of bone disease in solid tumors and multiple myeloma. Future Oncol (London, England). 2018;14:195–203.
Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132–9.
Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29:1125–32.
Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet (London, England). 2011;377:813–22.
Raje N, Terpos E, Willenbacher W, Shimizu K, Garcia-Sanz R, Durie B, et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018;19:370–81.
Henry D, Vadhan-Raj S, Hirsh V, von Moos R, Hungria V, Costa L, et al. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support Care Cancer. 2014;22:679–87.
Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading Pain severity by its interference with function. Pain. 1995;61:277–84.
Cleeland CS, Nakamura Y, Mendoza TR, Edwards KR, Douglas J, Serlin RC. Dimensions of the impact of cancer pain in a four country sample: new information from multidimensional scaling. Pain. 1996;67:1–273.
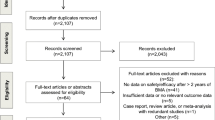
Moher D, Liberati A, Tetzlaff J, Altman DG, P. Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–41.
Vadhan-Raj S, von Moos R, Fallowfield LJ, Patrick DL, Goldwasser F, Cleeland CS, et al. Clinical benefit in patients with metastatic bone disease: results of a phase 3 study of denosumab versus zoledronic acid. Ann Oncol. 2012;23:3045–51.
Cleeland CS, Body JJ, Stopeck A, von Moos R, Fallowfield L, Mathias SD, et al. Pain outcomes in patients with advanced breast cancer and bone metastases: results from a randomized, double-blind study of denosumab and zoledronic acid. Cancer. 2013;119:832–8.
Sun L, Yu SY. Efficacy and safety of denosumab versus zoledronic acid in patients with bone metastases a systematic review and meta-analysis. Am J Clin Onco Cancer Clin Trials. 2013;36:399–403.
Zheng GZ, Chang B, Lin FX, Xie D, Hu QX, Yu GY, et al. Meta-analysis comparing denosumab and zoledronic acid for treatment of bone metastases in patients with advanced solid tumours. Eur J Cancer Care. 2017;26:e12541.
Ford JA, Jones R, Elders A, Mulatero C, Royle P, Sharma P, et al. Denosumab for treatment of bone metastases secondary to solid tumours: systematic review and network meta-analysis. Eur J Cancer. 2013;49:416–30.
Scagliotti G, Woll P, Manegold C, Solal-Celigny P, Lipton L, García-Sáenz JA, et al. Effect of denosumab versus zoledronic acid on overall survival in patients with lung cancer and bone metastases: results from a randomized phase 3 study. J Thorac Oncol. 2012;7:S61–2.
Udagawa H, Niho S, Kirita K, Umemura S, Matsumoto S, Yoh K, et al. Impact of denosumab use on the survival of untreated non-squamous non-small cell lung cancer patients with bone metastases. J Cancer Res Clin Oncol. 2017;143:1075–82.
Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I, et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440:692–6.
Raje N, Vadhan-Raj S, Willenbacher W, Terpos E, Hungria V, Spencer A, et al. Evaluating results from the multiple myeloma patient subset treated with denosumab or zoledronic acid in a randomized phase 3 trial. Blood Cancer J. 2016;6:e378.
von Moos R, Body JJ, Egerdie B, Stopeck A, Brown JE, Damyanov D, et al. Pain and health-related quality of life in patients with advanced solid tumours and bone metastases: integrated results from three randomized, double-blind studies of denosumab and zoledronic acid. Support Care Cancer. 2013;21:3497–507.
Martin M, Bell R, Bourgeois H, Brufsky A, Diel I, Eniu A, et al. Bone-related complications and quality of life in advanced breast cancer: results from a randomized phase III trial of denosumab versus zoledronic acid. Clin Cancer Res. 2012;18:4841–9.
Peddi P, Lopez-Olivo MA, Pratt GF, Suarez-Almazor ME. Denosumab in patients with cancer and skeletal metastases: a systematic review and meta-analysis. Cancer Treat Rev. 2013;39:97–104.
Smith MR, Egerdie B, Toriz NH, Feldman R, Tammela TLJ, Saad F, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745–55.
Terpos E, Szydlo R, Apperley JF, Hatjiharissi E, Politou M, Meletis J, et al. Soluble receptor activator of nuclear factor kappaB ligand-osteoprotegerin ratio predicts survival in multiple myeloma: proposal for a novel prognostic index. Blood. 2003;102:1064–9.
Menshawy A, Mattar O, Abdulkarim A, Kasem S, Nasreldin N, Menshawy E, et al. Denosumab versus bisphosphonates in patients with advanced cancers-related bone metastasis: systematic review and meta-analysis of randomized controlled trials. Support Care Cancer. 2018;26:1029–38.
von Moos R, Costa L, Gonzalez-Suarez E, Terpos E, Niepel D, Body JJ. Management of bone health in solid tumours: from bisphosphonates to a monoclonal antibody. Cancer Treat Rev. 2019;76:57–67.
Sun L, Yu S. Efficacy and safety of denosumab versus zoledronic acid in patients with bone metastases: a systematic review and meta-analysis. Am J Clin Oncol. 2013;36:399–403.
Higuchi T, Soga Y, Muro M, Kajizono M, Kitamura Y, Sendo T, et al. Replacing zoledronic acid with denosumab is a risk factor for developing osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:547–51.
Nicolatou-Galitis O, Schiodt M, Mendes RA, Ripamonti C, Hope S, Drudge-Coates L, et al. Medication-related osteonecrosis of the jaw: definition and best practice for prevention, diagnosis, and treatment. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;127:117–35.
Shibahara T. Antiresorptive agent-related osteonecrosis of the jaw (ARONJ): a twist of fate in the bone. Tohoku J Exp Med. 2019;247:75–86.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
There was no funding support of this work.
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, J., Zhou, L., Liu, X. et al. Meta-analysis of clinical trials to assess denosumab over zoledronic acid in bone metastasis. Int J Clin Pharm 43, 2–10 (2021). https://doi.org/10.1007/s11096-020-01105-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-020-01105-1