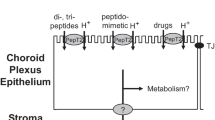
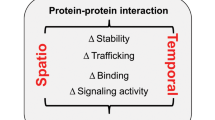
Abstract
The oligopeptide/histidine transporters PHT1 and PHT2, two mammalian solute carrier family 15A proteins, mediate the transmembrane transport of histidine and some di/tripeptides via proton gradient. PHT1 and PHT2 are distributed in a variety of tissues but are preferentially expressed in immune cells and localize to the lysosome-related organelles. Studies have reported the relationships between PHT1/PHT2 and immune diseases. PHT1 and PHT2 participate in the regulation of lysosomal homeostasis and lysosome-associated signaling pathways through their transport and nontransport functions, playing important roles in inflammatory diseases. In this review, we summarize recent research on PHT1 and PHT2, aiming to provide reference for their further biological research and as targets for drug design.


Similar content being viewed by others
References
Smith DE, Clémençon B, Hediger MA. Proton-coupled oligopeptide transporter family SLC15: Physiological, pharmacological and pathological implications. Mol Aspects Med. 2013;34:323–36.
Viennois E, Pujada A, Zen J, Merlin D. Function, Regulation, and Pathophysiological Relevance of the POT Superfamily, Specifically PepT1 in Inflammatory Bowel Disease. Compr Physiol. 2018;8:731–60.
Nakamura N, Lill JR, Phung Q, Jiang Z, Bakalarski C, de Maziere A, et al. Endosomes are specialized platforms for bacterial sensing and NOD2 signalling. Nature. 2014;509:240–4.
Sakata K, Yamashita T, Maeda M, Moriyama Y, Shimada S, Tohyama M. Cloning of a lymphatic peptide/histidine transporter. Biochem J. 2001;356:53–60.
Yamashita T, Shimada S, Guo W, Sato K, Kohmura E, Hayakawa T, et al. Cloning and functional expression of a brain peptide/histidine transporter. J Biol Chem. 1997;272:10205–11.
Herrera-Ruiz D, Wang Q, Gudmundsson OS, Cook TJ, Smith RL, Faria TN, et al. Spatial expression patterns of peptide transporters in the human and rat gastrointestinal tracts, Caco-2 in vitro cell culture model, and multiple human tissues. AAPS PharmSci. 2001;3:E9.
Bhardwaj RK, Herrera-Ruiz D, Eltoukhy N, Saad M, Knipp GT. The functional evaluation of human peptide/histidine transporter 1 (hPHT1) in transiently transfected COS-7 cells. Eur J Pharm Sci. 2006;27:533–42.
Botka CW, Wittig TW, Graul RC, Nielsen CU, Higaka K, Amidon GL, et al. Human proton/oligopeptide transporter (POT) genes: identification of putative human genes using bioinformatics. AAPS PharmSci. 2000;2:E16.
Mazzei A, Serino G, Romano A, Piccinno E, Scalavino V, Valentini AM, et al. Identification of SLC15A4/PHT1 Gene Products Upregulation Marking the Intestinal Epithelial Monolayer of Ulcerative Colitis Patients. Int J Mol Sci. 2022;23:13170.
Tai W, Chen Z, Cheng K. Expression profile and functional activity of peptide transporters in prostate cancer cells. Mol Pharm. 2013;10:477–87.
Sun D, Wang Y, Tan F, Fang D, Hu Y, Smith DE, et al. Functional and molecular expression of the proton-coupled oligopeptide transporters in spleen and macrophages from mouse and human. Mol Pharm. 2013;10:1409–16.
Wang Y, Sun D, Song F, Hu Y, Smith DE, Jiang H. Expression and regulation of the proton-coupled oligopeptide transporter PhT2 by LPS in macrophages and mouse spleen. Mol Pharm. 2014;11:1880–8.
He L, Wang B, Li Y, Zhu L, Li P, Zou F, et al. The Solute Carrier Transporter SLC15A3 Participates in Antiviral Innate Immune Responses against Herpes Simplex Virus-1. J Immunol Res. 2018;2018:5214187.
Sasawatari S, Okamura T, Kasumi E, Tanaka-Furuyama K, Yanobu-Takanashi R, Shirasawa S, et al. The solute carrier family 15A4 regulates TLR9 and NOD1 functions in the innate immune system and promotes colitis in mice. Gastroenterology. 2011;140:1513–25.
Ocheltree SM, Keep RF, Shen H, Yang D, Hughes BA, Smith DE. Preliminary investigation into the expression of proton-coupled oligopeptide transporters in neural retina and retinal pigment epithelium (RPE): lack of functional activity in RPE plasma membranes. Pharm Res. 2003;20:1364–72.
Lee J, Tattoli I, Wojtal KA, Vavricka SR, Philpott DJ, Girardin SE. pH-dependent internalization of muramyl peptides from early endosomes enables Nod1 and Nod2 signaling. J Biol Chem. 2009;284:23818–29.
Carl SM, Lindley DJ, Das D, Couraud PO, Weksler BB, Romero I, et al. ABC and SLC transporter expression and proton oligopeptide transporter (POT) mediated permeation across the human blood–brain barrier cell line, hCMEC/D3 [corrected]. Mol Pharm. 2010;7:1057–68.
Sun D, Tan F, Fang D, Wang Y, Zeng S, Jiang H. Expression of proton-coupled oligopeptide transporter (POTs) in prostate of mice and patients with benign prostatic hyperplasia (BPH) and prostate cancer (PCa). Prostate. 2013;73:287–95.
Lee CL, Huang CJ, Yang SH, Chang CC, Huang CC, Chien CC, et al. Discovery of genes from feces correlated with colorectal cancer progression. Oncol Lett. 2016;12:3378–84.
Vanaki N, Golmohammadi T, Jamshidi A, Akhtari M, Vojdanian M, Mostafaei S, et al. Increased inflammatory responsiveness of peripheral blood mononuclear cells (PBMCs) to in vitro NOD2 ligand stimulation in patients with ankylosing spondylitis. Immunopharmacol Immunotoxicol. 2018;40:393–400.
Oppermann H, Heinrich M, Birkemeyer C, Meixensberger J, Gaunitz F. The proton-coupled oligopeptide transporters PEPT2, PHT1 and PHT2 mediate the uptake of carnosine in glioblastoma cells. Amino Acids. 2019;51:999–1008.
Blasius AL, Arnold CN, Georgel P, Rutschmann S, Xia Y, Lin P, et al. Slc15a4, AP-3, and Hermansky-Pudlak syndrome proteins are required for Toll-like receptor signaling in plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2010;107:19973–8.
Hu Y, Xie Y, Keep RF, Smith DE. Divergent developmental expression and function of the proton-coupled oligopeptide transporters PepT2 and PhT1 in regional brain slices of mouse and rat. J Neurochem. 2014;129:955–65.
Kitakaze T, Sakamoto T, Kitano T, Inoue N, Sugihara F, Harada N, et al. The collagen derived dipeptide hydroxyprolyl-glycine promotes C2C12 myoblast differentiation and myotube hypertrophy. Biochem Biophys Res Commun. 2016;478:1292–7.
Kobayashi T, Tsutsui H, Shimabukuro-Demoto S, Yoshida-Sugitani R, Karyu H, Furuyama-Tanaka K, et al. Lysosome biogenesis regulated by the amino-acid transporter SLC15A4 is critical for functional integrity of mast cells. Int Immunol. 2017;29:551–66.
Yuizumi N, Harada Y, Kuniya T, Sunabori T, Koike M, Wakabayashi M, et al. Maintenance of neural stem-progenitor cells by the lysosomal biosynthesis regulators TFEB and TFE3 in the embryonic mouse telencephalon. Stem Cells. 2021;39:929–44.
Romano A, Barca A, Kottra G, Daniel H, Storelli C, Verri T. Functional expression of SLC15 peptide transporters in rat thyroid follicular cells. Mol Cell Endocrinol. 2010;315:174–81.
Soga M, Ohashi A, Taniguchi M, Matsui T, Tsuda T. The di-peptide Trp-His activates AMP-activated protein kinase and enhances glucose uptake independently of insulin in L6 myotubes. FEBS Open Bio. 2014;4:898–904.
Barca A, Ippati S, Urso E, Vetrugno C, Storelli C, Maffia M, et al. Carnosine modulates the Sp1-Slc31a1/Ctr1 copper-sensing system and influences copper homeostasis in murine CNS-derived cells. Am J Physiol Cell Physiol. 2019;316:C235–45.
Huang Q, Vera Delgado JM, Seni Pinoargote OD, Llaguno RA. Molecular evolution of the Slc15 family and its response to waterborne copper and mercury exposure in tilapia. Aquat Toxicol. 2015;163:140–7.
Saikrithi P, Balasubramanian CP, Otta SK, Tomy S. Expression dynamics of solute carrier family 15 member 4 (SLC15A4) and its potential regulatory role in ovarian development of the Indian white shrimp Penaeus indicus. Mol Biol Rep. 2020;47:3797–805.
Song F, Yi Y, Li C, Hu Y, Wang J, Smith DE, et al. Regulation and biological role of the peptide/histidine transporter SLC15A3 in Toll-like receptor-mediated inflammatory responses in macrophage. Cell Death Dis. 2018;9:770.
Wang Y, Hu Y, Li P, Weng Y, Kamada N, Jiang H, et al. Expression and regulation of proton-coupled oligopeptide transporters in colonic tissue and immune cells of mice. Biochem Pharmacol. 2018;148:163–73.
Groebner AE, Rubio-Aliaga I, Schulke K, Reichenbach HD, Daniel H, Wolf E, et al. Increase of essential amino acids in the bovine uterine lumen during preimplantation development. Reproduction. 2011;141:685–95.
Song F, Hu Y, Wang Y, Smith DE, Jiang H. Functional Characterization of Human Peptide/Histidine Transporter 1 in Stably Transfected MDCK Cells. Mol Pharm. 2018;15:385–93.
Heinz LX, Lee J, Kapoor U, Kartnig F, Sedlyarov V, Papakostas K, et al. TASL is the SLC15A4-associated adaptor for IRF5 activation by TLR7-9. Nature. 2020;581:316–22.
Kobayashi T, Nguyen-Tien D, Ohshima D, Karyu H, Shimabukuro-Demoto S, Yoshida-Sugitani R, et al. Human SLC15A4 is crucial for TLR-mediated type I interferon production and mitochondrial integrity. Int Immunol. 2021;33:399–406.
.Rimann I, Gonzalez-Quintial R, Baccala R, Kiosses W B, Teijaro J R, Parker C G, et al. The solute carrier SLC15A4 is required for optimal trafficking of nucleic acid-sensing TLRs and ligands to endolysosomes. Proc Natl Acad Sci U S A, 2022, 119: e2200544119.
Han JW, Zheng HF, Cui Y, Sun LD, Ye DQ, Hu Z, et al. Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat Genet. 2009;41:1234–7.
Griffith AD, Zaidi AK, Pietro A, Hadiono M, Yang JS, Davis R, et al. A requirement for slc15a4 in imiquimod-induced systemic inflammation and psoriasiform inflammation in mice. Sci Rep. 2018;8:14451.
Wang Y, Li P, Song F, Yang X, Weng Y, Ma Z, et al. Substrate Transport Properties of the Human Peptide/Histidine Transporter PHT2 in Transfected MDCK Cells. J Pharm Sci. 2019;108:3416–24.
Kalatzis V, Cherqui S, Antignac C, Gasnier B. Cystinosin, the protein defective in cystinosis, is a H(+)-driven lysosomal cystine transporter. EMBO J. 2001;20:5940–9.
Scalise M, Pochini L, Giangregorio N, Tonazzi A, Indiveri C. Proteoliposomes as tool for assaying membrane transporter functions and interactions with xenobiotics. Pharmaceutics. 2013;5:472–97.
Rebsamen M, Pochini L, Stasyk T, de Araujo ME, Galluccio M, Kandasamy RK, et al. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature. 2015;519:477–81.
Cheng L, Deepak R, Wang G, Meng Z, Tao L, Xie M, et al. Hepatic mitochondrial NAD + transporter SLC25A47 activates AMPKalpha mediating lipid metabolism and tumorigenesis. Hepatology, 2023, ( print ).
Li S, Wu Q, Jiang Z, Wu Y, Li Y, Ni B, et al. miR-31-5p Regulates Type I Interferon by Targeting SLC15A4 in Plasmacytoid Dendritic Cells of Systemic Lupus Erythematosus. J Inflamm Res. 2022;15:6607–16.
Huang H, Wang J, Chen S, He H, Shang Y, Guo X, et al. SLC15A4 Serves as a Novel Prognostic Biomarker and Target for Lung Adenocarcinoma. Front Genet. 2021;12:666607.
Buteyn NJ, Santhanam R, Merchand-Reyes G, Murugesan RA, Dettorre GM, Byrd JC, et al. Activation of the Intracellular Pattern Recognition Receptor NOD2 Promotes Acute Myeloid Leukemia (AML) Cell Apoptosis and Provides a Survival Advantage in an Animal Model of AML. J Immunol. 2020;204:1988–97.
Holecek M. Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement. Nutrients. 2020;12:848.
Kobayashi T, Shimabukuro-Demoto S, Yoshida-Sugitani R, Furuyama-Tanaka K, Karyu H, Sugiura Y, et al. The histidine transporter SLC15A4 coordinates mTOR-dependent inflammatory responses and pathogenic antibody production. Immunity. 2014;41:375–88.
Hu Y, Song F, Jiang H, Nunez G, Smith DE. SLC15A2 and SLC15A4 Mediate the Transport of Bacterially Derived Di/Tripeptides To Enhance the Nucleotide-Binding Oligomerization Domain-Dependent Immune Response in Mouse Bone Marrow-Derived Macrophages. J Immunol. 2018;201:652–62.
Pelka K, Shibata T, Miyake K, Latz E. Nucleic acid-sensing TLRs and autoimmunity: novel insights from structural and cell biology. Immunol Rev. 2016;269:60–75.
Baccala R, Gonzalez-Quintial R, Blasius AL, Rimann I, Ozato K, Kono DH, et al. Essential requirement for IRF8 and SLC15A4 implicates plasmacytoid dendritic cells in the pathogenesis of lupus. Proc Natl Acad Sci U S A. 2013;110:2940–5.
Kobayashi T, Nguyen-Tien D, Sorimachi Y, Sugiura Y, Suzuki T, Karyu H, et al. SLC15A4 mediates M1-prone metabolic shifts in macrophages and guards immune cells from metabolic stress. Proc Natl Acad Sci U S A. 2021;118:e2100295118.
Lopez-Haber C, Netting DJ, Hutchins Z, Ma X, Hamilton KE, Mantegazza AR. The phagosomal solute transporter SLC15A4 promotes inflammasome activity via mTORC1 signaling and autophagy restraint in dendritic cells. EMBO J. 2022;41:e111161.
Kiriakidou M, Ching C L. Systemic Lupus Erythematosus. Ann Intern Med, 2020, 172: ITC81-ITC96.
Lee HS, Kim T, Bang SY, Na YJ, Kim I, Kim K, et al. Ethnic specificity of lupus-associated loci identified in a genome-wide association study in Korean women. Ann Rheum Dis. 2014;73:1240–5.
Wang C, Ahlford A, Jarvinen TM, Nordmark G, Eloranta ML, Gunnarsson I, et al. Genes identified in Asian SLE GWASs are also associated with SLE in Caucasian populations. Eur J Hum Genet. 2013;21:994–9.
He CF, Liu YS, Cheng YL, Gao JP, Pan TM, Han JW, et al. TNIP1, SLC15A4, ETS1, RasGRP3 and IKZF1 are associated with clinical features of systemic lupus erythematosus in a Chinese Han population. Lupus. 2010;19:1181–6.
Zuo XB, Sheng YJ, Hu SJ, Gao JP, Li Y, Tang HY, et al. Variants in TNFSF4, TNFAIP3, TNIP1, BLK, SLC15A4 and UBE2L3 interact to confer risk of systemic lupus erythematosus in Chinese population. Rheumatol Int. 2014;34:459–64.
Zhang M, Chen F, Zhang D, Zhai Z, Hao F. Association Study Between SLC15A4 Polymorphisms and Haplotypes and Systemic Lupus Erythematosus in a Han Chinese Population. Genet Test Mol Biomarkers. 2016;20:451–8.
Skopelitou D, Srivastava A, Miao B, Kumar A, Dymerska D, Paramasivam N, et al. Whole exome sequencing identifies novel germline variants of SLC15A4 gene as potentially cancer predisposing in familial colorectal cancer. Mol Genet Genomics. 2022;297:965–79.
Takeuchi F, Ochiai Y, Serizawa M, Yanai K, Kuzuya N, Kajio H, et al. Search for type 2 diabetes susceptibility genes on chromosomes 1q, 3q and 12q. J Hum Genet. 2008;53:314–24.
Simm F, Griesbeck A, Choukair D, Weiss B, Paramasivam N, Klammt J, et al. Identification of SLC20A1 and SLC15A4 among other genes as potential risk factors for combined pituitary hormone deficiency. Genet Med. 2018;20:728–36.
Ibragimova I, Ibanez de Caceres I, Hoffman AM, Potapova A, Dulaimi E, Al-Saleem T, et al. Global reactivation of epigenetically silenced genes in prostate cancer. Cancer Prev Res (Phila). 2010;2010(3):1084–92.
Nagata H, Kozaki KI, Muramatsu T, Hiramoto H, Tanimoto K, Fujiwara N, et al. Genome-wide screening of DNA methylation associated with lymph node metastasis in esophageal squamous cell carcinoma. Oncotarget. 2017;8:37740–50.
Buttarelli M, Ciucci A, Palluzzi F, Raspaglio G, Marchetti C, Perrone E, et al. Identification of a novel gene signature predicting response to first-line chemotherapy in BRCA wild-type high-grade serous ovarian cancer patients. J Exp Clin Cancer Res. 2022;41:50.
Zhou D, Yang L, Zheng L, Ge W, Li D, Zhang Y, et al. Exome capture sequencing of adenoma reveals genetic alterations in multiple cellular pathways at the early stage of colorectal tumorigenesis. PLoS One. 2013;8:e53310.
Franca JA, Gayden T, Bareke E, Santos JN, de Sousa SF, Bastos-Rodrigues L, et al. Whole-exome sequencing reveals novel vacuolar ATPase genes’ variants and variants in genes involved in lysosomal biology and autophagosomal formation in oral granular cell tumors. J Oral Pathol Med. 2021;50:410–7.
Killer M, Wald J, Pieprzyk J, Marlovits TC, Low C. Structural snapshots of human PepT1 and PepT2 reveal mechanistic insights into substrate and drug transport across epithelial membranes. Sci Adv. 2021;7(45):eabk3259.
Funding
This work was supported by the National Natural Science Foundation of China (82073929), and by the Natural Science Foundation of Zhejiang Province (LQ23H310030, LZ23H030001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Minlei Dong and Ping Li are co-first authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dong, M., Li, P., Luo, J. et al. Oligopeptide/Histidine Transporter PHT1 and PHT2 — Function, Regulation, and Pathophysiological Implications Specifically in Immunoregulation. Pharm Res 40, 2585–2596 (2023). https://doi.org/10.1007/s11095-023-03589-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03589-8