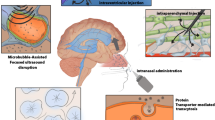
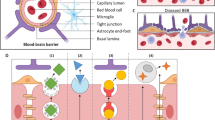
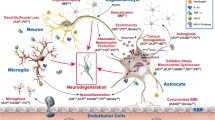
Abstract
Nucleic acid-based therapeutic molecules including small interfering RNA (siRNA), microRNA(miRNA), antisense oligonucleotides (ASOs), messenger RNA (mRNA), and DNA-based gene therapy have tremendous potential for treating diseases in the central nervous system (CNS). However, achieving clinically meaningful delivery to the brain and particularly to target cells and sub-cellular compartments is typically very challenging. Mediating cell-specific delivery in the CNS would be a crucial advance that mitigates off-target effects and toxicities. In this review, we describe these challenges and provide contemporary evidence of advances in cellular and sub-cellular delivery using a variety of delivery mechanisms and alternative routes of administration, including the nose-to-brain approach. Strategies to achieve subcellular localization, endosomal escape, cytosolic bioavailability, and nuclear transfer are also discussed. Ultimately, there are still many challenges to translating these experimental strategies into effective and clinically viable approaches for treating patients.
Graphical Abstract



Similar content being viewed by others
Abbreviations
- AAV:
-
Adeno-associated virus
- AD:
-
Alzheimer’s disease
- ALS:
-
Amyotrophic lateral sclerosis
- ASOs:
-
Antisense oligonucleotides
- bp:
-
Base pairs
- BBB:
-
Blood-brain barrier
- BCSFB:
-
Blood cerebrospinal-fluid barrier
- BDNF:
-
Brain-derived neurotrophic factor
- CPP:
-
Cell-penetrating peptides
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- CRISPR:
-
Clustered regularly interspaced short palindromic repeats
- CED:
-
Convection-enhanced delivery
- Cas9:
-
CRISPR-associated protein 9
- GDNF:
-
Glial cell-derived neurotrophic factor
- GFAP:
-
Glial fibrillary acidic protein
- HTT:
-
Huntingtin
- HD:
-
Huntington’s disease
- ICV:
-
Intracerebroventricular
- IP:
-
Intraparenchymal
- IT-CM:
-
Intrathecal—cisterna magna
- IT-L:
-
Intrathecal – lumbar
- IT:
-
Intrathecal
- IV:
-
Intravenous
- kDa:
-
Kilodalton
- LNPs:
-
Lipid nanoparticles
- MegNs:
-
Meganucleases
- mRNA:
-
Messenger RNA
- miRNA:
-
MicroRNA
- MIND:
-
Minimally-Invasive Nasal Depot
- MS:
-
Multiple sclerosis
- MSA:
-
Multiple system atrophy
- NPC:
-
Nuclear pore complex
- ODN:
-
Oligonucleotide
- PD:
-
Parkinson’s disease
- PEI:
-
Polyethylenimine
- RVG:
-
Rabies virus glycoprotein
- shRNA:
-
Short hairpin RNA
- siRNA:
-
Short interfering RNA
- SMA:
-
Spinal muscular atrophy
- SOD1:
-
Superoxide dismutase 1
- TALENs:
-
Transcription activator-like effector nuclease
- TfR:
-
Transferrin receptor
- ZFNs:
-
Zinc finger nucleases
References
Simonato M, Bennett J, Boulis NM, Castro MG, Fink DJ, Goins WF, et al. Progress in gene therapy for neurological disorders. Nat Rev Neurol. 2013;9(5):277–91.
Lima Giacobbo B, Doorduin J, Klein HC, Dierckx R, Bromberg E, de Vries EFJ. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol Neurobiol. 2019;56(5):3295–312.
Nagabhushan Kalburgi S, Khan NN, Gray SJ. Recent gene therapy advancements for neurological diseases. Discov Med. 2013;15(81):111–9.
Piguet F, Alves S, Cartier N. Clinical Gene Therapy for Neurodegenerative Diseases: Past, Present, and Future. Hum Gene Ther. 2017;28(11):988–1003.
Cicalese MP, Aiuti A. New perspectives in gene therapy for inherited disorders. Pediatr Allergy Immunol. 2020;31(Suppl 24):5–7.
Sung YK, Kim SW. Recent advances in the development of gene delivery systems. Biomater Res. 2019;23(1):8.
Bulaklak K, Gersbach CA. The once and future gene therapy. Nat Commun. 2020;11(1):5820.
Papanikolaou E, Bosio A. The Promise and the Hope of Gene Therapy. Front Genome Ed. 2021;3:618346.
Pan X, Veroniaina H, Su N, Sha K, Jiang F, Wu Z, et al. Applications and developments of gene therapy drug delivery systems for genetic diseases. Asian J Pharm Sci. 2021;16(6):687–703.
Alagoz M, Kherad N. Advance genome editing technologies in the treatment of human diseases: CRISPR therapy (Review). Int J Mol Med. 2020;46(2):521–34.
Yamada Y. Nucleic Acid Drugs-Current Status, Issues, and Expectations for Exosomes. Cancers. 2021;13(19):5002.
Mendes BB, Conniot J, Avital A, Yao D, Jiang X, Zhou X, et al. Nanodelivery of nucleic acids. Nature Rev Methods Primers. 2022;2:24.
Khalil AM. The genome editing revolution: review. J Genet Eng Biotechnol. 2020;18(1):68.
Pezzoli D, Chiesa R, De Nardo L, Candiani G. We still have a long way to go to effectively deliver genes! J Appl Biomater Funct Mater. 2012;10(2):82–91.
Klug B, Celis P, Carr M, Reinhardt J. Regulatory structures for gene therapy medicinal products in the European Union. Methods Enzymol. 2012;507:337–54.
Paunovska K, Loughrey D, Dahlman JE. Drug delivery systems for RNA therapeutics. Nat Rev Genet. 2022;23(5):265–80.
Deverman BE, Ravina BM, Bankiewicz KS, Paul SM, Sah DWY. Gene therapy for neurological disorders: progress and prospects. Nat Rev Drug Discovery. 2018;17(9):641–59.
Bobbin ML, Rossi JJ. RNA Interference (RNAi)-Based Therapeutics: Delivering on the Promise? Annu Rev Pharmacol Toxicol. 2016;56:103–22.
Lorenzer C, Dirin M, Winkler AM, Baumann V, Winkler J. Going beyond the liver: progress and challenges of targeted delivery of siRNA therapeutics. J Control Release. 2015;203:1–15.
Havens MA, Hastings ML. Splice-switching antisense oligonucleotides as therapeutic drugs. Nucleic Acids Res. 2016;44(14):6549–63.
Sridharan K, Gogtay NJ. Therapeutic nucleic acids: current clinical status. Br J Clin Pharmacol. 2016;82(3):659–72.
Geary RS, Norris D, Yu R, Bennett CF. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv Drug Deliv Rev. 2015;87:46–51.
DeVos SL, Miller RL, Schoch KM, Holmes BB, Kebodeaux CS, Wegener A, et al. Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Sci Transl Med. 2017;9(374):eaag0481.
Höck J, Meister G. The Argonaute protein family. Genome Biol. 2008;9(2):210.
Liu Y, Guo Y, An S, Kuang Y, He X, Ma H, et al. Targeting caspase-3 as dual therapeutic benefits by RNAi facilitating brain-targeted nanoparticles in a rat model of Parkinson’s disease. PLoS One. 2013;8(5):e62905.
Lu KW, Chen ZY, Hou TS. Protective effect of liposome-mediated glial cell line-derived neurotrophic factor gene transfer in vivo on motoneurons following spinal cord injury in rats. Chin J Traumatol = Zhonghua chuang shang za zhi. 2004;7(5):275–9.
Alarcón-Arís D, Pavia-Collado R, Miquel L, Cóppola V, Ferrés-Coy A, Ruiz-Bronchal E, et al. Anti-α-synuclein ASO delivered to monoamine neurons prevents α-synuclein accumulation in a Parkinson’s disease-like mouse model and in monkeys. EBioMedicine. 2020;59:102944.
Wong E, Liao GP, Chang JC, Xu P, Li YM, Greengard P. GSAP modulates γ-secretase specificity by inducing conformational change in PS1. Proc Natl Acad Sci USA. 2019;116(13):6385–90.
Yoon HH, Ye S, Lim S, Jo A, Lee H, Hong F, et al. CRISPR-Cas9 Gene Editing Protects from the A53T-SNCA Overexpression-Induced Pathology of Parkinson’s Disease In Vivo. The CRISPR journal. 2022;5(1):95–108.
Niu S, Zhang L-K, Zhang L, Zhuang S, Zhan X, Chen W-Y, et al. Inhibition by Multifunctional Magnetic Nanoparticles Loaded with Alpha-Synuclein RNAi Plasmid in a Parkinson’s Disease Model. Theranostics. 2017;7(2):344–56.
Gao Y, Wang Z-Y, Zhang J, Zhang Y, Huo H, Wang T, et al. RVG-Peptide-Linked Trimethylated Chitosan for Delivery of siRNA to the Brain. Biomacromol. 2014;15(3):1010–8.
Galloway DA, Blandford SN, Berry T, Williams JB, Stefanelli M, Ploughman M, et al. miR-223 promotes regenerative myeloid cell phenotype and function in the demyelinated central nervous system. Glia. 2019;67(5):857–69.
Jimenez-Mateos EM, Engel T, Merino-Serrais P, Fernaud-Espinosa I, Rodriguez-Alvarez N, Reynolds J, et al. Antagomirs targeting microRNA-134 increase hippocampal pyramidal neuron spine volume in vivo and protect against pilocarpine-induced status epilepticus. Brain Struct Funct. 2015;220(4):2387–99.
Lin C-Y, Perche F, Ikegami M, Uchida S, Kataoka K, Itaka K. Messenger RNA-based therapeutics for brain diseases: An animal study for augmenting clearance of beta-amyloid by intracerebral administration of neprilysin mRNA loaded in polyplex nanomicelles. J Control Release. 2016;235:268–75.
Edinger D, Wagner E. Bioresponsive polymers for the delivery of therapeutic nucleic acids. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3(1):33–46.
Vaseghi G, Rafiee L, Javanmard SH. Non-viral Delivery Systems for Breast Cancer Gene Therapy. Curr Gene Ther. 2017;17(2):147–53.
Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15(8):541–55.
Crawford L, Rosch J, Putnam D. Concepts, technologies, and practices for drug delivery past the blood-brain barrier to the central nervous system. J Control Release. 2016;240:251–66.
Fumoto S, Yamamoto T, Okami K, Maemura Y, Terada C, Yamayoshi A, et al. Understanding In Vivo Fate of Nucleic Acid and Gene Medicines for the Rational Design of Drugs. Pharmaceutics. 2021;13(2):159.
Luissint AC, Artus C, Glacial F, Ganeshamoorthy K, Couraud PO. Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation. Fluids Barriers CNS. 2012;9(1):23.
Dias N, Stein CA. Antisense oligonucleotides: basic concepts and mechanisms. Mol Cancer Ther. 2002;1(5):347–55.
Layzer JM, McCaffrey AP, Tanner AK, Huang Z, Kay MA, Sullenger BA. In vivo activity of nuclease-resistant siRNAs. RNA (New York, NY). 2004;10(5):766–71.
Khvorova A, Watts JK. The chemical evolution of oligonucleotide therapies of clinical utility. Nat Biotechnol. 2017;35(3):238–48.
Wan WB, Seth PP. The Medicinal Chemistry of Therapeutic Oligonucleotides. J Med Chem. 2016;59(21):9645–67.
Boo SH, Kim YK. The emerging role of RNA modifications in the regulation of mRNA stability. Exp Mol Med. 2020;52(3):400–8.
Corey DR. Chemical modification: the key to clinical application of RNA interference? J Clin Investig. 2007;117(12):3615–22.
Lukacs GL, Haggie P, Seksek O, Lechardeur D, Freedman N, Verkman AS. Size-dependent DNA mobility in cytoplasm and nucleus. J Biol Chem. 2000;275(3):1625–9.
Suh J, Dawson M, Hanes J. Real-time multiple-particle tracking: applications to drug and gene delivery. Adv Drug Deliv Rev. 2005;57(1):63–78.
Vázquez E, Ferrer-Miralles N, Villaverde A. Peptide-assisted traffic engineering for nonviral gene therapy. Drug Discovery Today. 2008;13(23):1067–74.
Wong SY, Pelet JM, Putnam D. Polymer systems for gene delivery—Past, present, and future. Prog Polym Sci. 2007;32(8):799–837.
Prasuhn J, Brüggemann N. Gene Therapeutic Approaches for the Treatment of Mitochondrial Dysfunction in Parkinson's Disease. Genes. 2021;12(11):1840.
Morris JA, Boshoff CH, Schor NF, Wong LM, Gao G, Davidson BL. Next-generation strategies for gene-targeted therapies of central nervous system disorders: A workshop summary. Mol Ther. 2021;29(12):3332–44.
Hsu P-H, Wei K-C, Huang C-Y, Wen C-J, Yen T-C, Liu C-L, et al. Noninvasive and Targeted Gene Delivery into the Brain Using Microbubble-Facilitated Focused Ultrasound. PLoS ONE. 2013;8(2):e57682.
Wang PP, Frazier J, Brem H. Local drug delivery to the brain. Adv Drug Deliv Rev. 2002;54(7):987–1013.
Sadekar SS, Bowen M, Cai H, Jamalian S, Rafidi H, Shatz-Binder W, et al. Translational Approaches for Brain Delivery of Biologics via Cerebrospinal Fluid. Clin Pharmacol Ther. 2022;111(4):826–34.
Perez BA, Shutterly A, Chan YK, Byrne BJ, Corti M. Management of Neuroinflammatory Responses to AAV-Mediated Gene Therapies for Neurodegenerative Diseases. Brain Sci. 2020;10(2):119.
Scarpa M, Bellettato CM, Lampe C, Begley DJ. Neuronopathic lysosomal storage disorders: Approaches to treat the central nervous system. Best Pract Res Clin Endocrinol Metab. 2015;29(2):159–71.
Alam MI, Beg S, Samad A, Baboota S, Kohli K, Ali J, et al. Strategy for effective brain drug delivery. Eur J Pharm Sci. 2010;40(5):385–403.
Yadav DB, Maloney JA, Wildsmith KR, Fuji RN, Meilandt WJ, Solanoy H, et al. Widespread brain distribution and activity following i.c.v. infusion of anti-β-secretase (BACE1) in nonhuman primates. Br J Pharmacol. 2017;174(22):4173–85.
Tan J-KY, Sellers DL, Pham B, Pun SH, Horner PJ. Non-Viral Nucleic Acid Delivery Strategies to the Central Nervous System. Front Mol Neurosci. 2016;9:108.
Pardridge WM. Drug transport in brain via the cerebrospinal fluid. Fluids Barriers CNS. 2011;8(1):7.
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci Transl Med. 2012;4(147):147ra111.
Harbaugh RE, Saunders RL, Reeder RF. Use of implantable pumps for central nervous system drug infusions to treat neurological disease. Neurosurgery. 1988;23(6):693–8.
Scheld WM. Drug delivery to the central nervous system: general principles and relevance to therapy for infections of the central nervous system. Rev Infect Dis. 1989;11(Suppl 7):S1669–90.
Bellettato CM, Scarpa M. Possible strategies to cross the blood–brain barrier. Ital J Pediatr. 2018;44(2):131.
Taghian T, Marosfoi MG, Puri AS, Cataltepe OI, King RM, Diffie EB, et al. A Safe and Reliable Technique for CNS Delivery of AAV Vectors in the Cisterna Magna. Mol Ther. 2020;28(2):411–21.
Hinderer C, Bell P, Katz N, Vite CH, Louboutin JP, Bote E, et al. Evaluation of Intrathecal Routes of Administration for Adeno-Associated Viral Vectors in Large Animals. Hum Gene Ther. 2018;29(1):15–24.
Hinderer C, Bell P, Vite CH, Louboutin JP, Grant R, Bote E, et al. Widespread gene transfer in the central nervous system of cynomolgus macaques following delivery of AAV9 into the cisterna magna. Mol Ther Methods Clin Dev. 2014;1:14051.
Wurster CD, Winter B, Wollinsky K, Ludolph AC, Uzelac Z, Witzel S, et al. Intrathecal administration of nusinersen in adolescent and adult SMA type 2 and 3 patients. J Neurol. 2019;266(1):183–94.
Calias P, Banks WA, Begley D, Scarpa M, Dickson P. Intrathecal delivery of protein therapeutics to the brain: A critical reassessment. Pharmacol Ther. 2014;144(2):114–22.
Samaranch L, Bringas J, Pivirotto P, Sebastian WS, Forsayeth J, Bankiewicz K. Cerebellomedullary Cistern Delivery for AAV-Based Gene Therapy: A Technical Note for Nonhuman Primates. Human Gene Ther Methods. 2016;27(1):13–6.
Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods. 2005;148(1):1–18.
Potts MB, Silvestrini MT, Lim DA. Devices for cell transplantation into the central nervous system: Design considerations and emerging technologies. Surg Neurol Int. 2013;4(Suppl 1):S22-30.
Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5(2):79–88.
Mehta AM, Sonabend AM, Bruce JN. Convection-Enhanced Delivery. Neurotherapeutics. 2017;14(2):358–71.
Chen MY, Lonser RR, Morrison PF, Governale LS, Oldfield EH. Variables affecting convection-enhanced delivery to the striatum: a systematic examination of rate of infusion, cannula size, infusate concentration, and tissue—cannula sealing time. J Neurosurg. 1999;90(2):315–20.
Monani UR. Spinal Muscular Atrophy: A Deficiency in a Ubiquitous Protein; a Motor Neuron-Specific Disease. Neuron. 2005;48(6):885–95.
Mitchell JD, Borasio GD. Amyotrophic lateral sclerosis. Lancet (London, England). 2007;369(9578):2031–41.
Ström AL, Gal J, Shi P, Kasarskis EJ, Hayward LJ, Zhu H. Retrograde axonal transport and motor neuron disease. J Neurochem. 2008;106(2):495–505.
D’Souza AA, Kutlehria S, Huang D, Bleier BS, Amiji MM. Nasal delivery of nanotherapeutics for CNS diseases: challenges and opportunities. Nanomedicine. 2021;16(30):2651–5.
Padmakumar S, Jones G, Khorkova O, Hsiao J, Kim J, Bleier BS, et al. Osmotic core-shell polymeric implant for sustained BDNF AntagoNAT delivery in CNS using minimally invasive nasal depot (MIND) approach. Biomaterials. 2021;276:120989.
Padmakumar S, Jones G, Pawar G, Khorkova O, Hsiao J, Kim J, et al. Minimally Invasive Nasal Depot (MIND) technique for direct BDNF AntagoNAT delivery to the brain. J Control Release. 2021;331:176–86.
Holm A, Hansen SN, Klitgaard H, Kauppinen S. Clinical advances of RNA therapeutics for treatment of neurological and neuromuscular diseases. RNA Biol. 2022;19(1):594–608.
Haché M, Swoboda KJ, Sethna N, Farrow-Gillespie A, Khandji A, Xia S, et al. Intrathecal Injections in Children With Spinal Muscular Atrophy: Nusinersen Clinical Trial Experience. J Child Neurol. 2016;31(7):899–906.
Bortolani S, Stura G, Ventilii G, Vercelli L, Rolle E, Ricci F, et al. Intrathecal administration of nusinersen in adult and adolescent patients with spinal muscular atrophy and scoliosis: Transforaminal versus conventional approach. Neuromuscul Dis NMD. 2019;29(10):742–6.
Cordts I, Lingor P, Friedrich B, Pernpeintner V, Zimmer C, Deschauer M, et al. Intrathecal nusinersen administration in adult spinal muscular atrophy patients with complex spinal anatomy. Ther Adv Neurol Disord. 2020;13:1756286419887616.
Jimenez-Mateos EM, Engel T, Merino-Serrais P, McKiernan RC, Tanaka K, Mouri G, et al. Silencing microRNA-134 produces neuroprotective and prolonged seizure-suppressive effects. Nat Med. 2012;18(7):1087–94.
Zhu D, Schieferecke AJ, Lopez PA, Schaffer DV. Adeno-Associated Virus Vector for Central Nervous System Gene Therapy. Trends Mol Med. 2021;27(6):524–37.
Roberts TC, Langer R, Wood MJA. Advances in oligonucleotide drug delivery. Nat Rev Drug Discovery. 2020;19(10):673–94.
Helmschrodt C, Höbel S, Schöniger S, Bauer A, Bonicelli J, Gringmuth M, et al. Polyethylenimine Nanoparticle-Mediated siRNA Delivery to Reduce α-Synuclein Expression in a Model of Parkinson’s Disease. Mol Ther Nucleic Acids. 2017;9:57–68.
Hirunagi T, Sahashi K, Tachikawa K, Leu AI, Nguyen M, Mukthavaram R, et al. Selective suppression of polyglutamine-expanded protein by lipid nanoparticle-delivered siRNA targeting CAG expansions in the mouse CNS. Mol Ther Nucleic Acids. 2021;24:1–10.
Nabhan JF, Wood KM, Rao VP, Morin J, Bhamidipaty S, LaBranche TP, et al. Intrathecal delivery of frataxin mRNA encapsulated in lipid nanoparticles to dorsal root ganglia as a potential therapeutic for Friedreich’s ataxia. Sci Rep. 2016;6(1):20019.
Godinho B, Gilbert JW, Haraszti RA, Coles AH, Biscans A, Roux L, et al. Pharmacokinetic Profiling of Conjugated Therapeutic Oligonucleotides: A High-Throughput Method Based Upon Serial Blood Microsampling Coupled to Peptide Nucleic Acid Hybridization Assay. Nucleic Acid Ther. 2017;27(6):323–34.
Nikan M, Osborn MF, Coles AH, Godinho BM, Hall LM, Haraszti RA, et al. Docosahexaenoic Acid Conjugation Enhances Distribution and Safety of siRNA upon Local Administration in Mouse Brain. Mol Ther Nucleic Acids. 2016;5(8):e344.
Nikan M, Osborn MF, Coles AH, Biscans A, Godinho BMDC, Haraszti RA, et al. Synthesis and Evaluation of Parenchymal Retention and Efficacy of a Metabolically Stable O-Phosphocholine-N-docosahexaenoyl-l-serine siRNA Conjugate in Mouse Brain. Bioconjug Chem. 2017;28(6):1758–66.
Brown KM, Nair JK, Janas MM, Anglero-Rodriguez YI, Dang LTH, Peng H, et al. Expanding RNAi therapeutics to extrahepatic tissues with lipophilic conjugates. Nat Biotechnol. 2022;40(10):1500–1508.
Das S, Mishra KP, Ganju L, Singh SB. Intranasally delivered small interfering RNA-mediated suppression of scavenger receptor Mac-1 attenuates microglial phenotype switching and working memory impairment following hypoxia. Neuropharmacology. 2018;137:240–55.
Rodriguez M, Lapierre J, Ojha CR, Kaushik A, Batrakova E, Kashanchi F, et al. Intranasal drug delivery of small interfering RNA targeting Beclin1 encapsulated with polyethylenimine (PEI) in mouse brain to achieve HIV attenuation. Sci Rep. 2017;7(1):1862.
Dhaliwal HK, Fan Y, Kim J, Amiji MM. Intranasal Delivery and Transfection of mRNA Therapeutics in the Brain Using Cationic Liposomes. Mol Pharm. 2020;17(6):1996–2005.
Sanchez-Ramos J, Song S, Kong X, Foroutan P, Martinez G, Dominguez-Viqueria W, et al. Chitosan-Mangafodipir nanoparticles designed for intranasal delivery of siRNA and DNA to brain. J Drug Deliv Sci Technol. 2018;43:453–60.
Kanazawa T, Akiyama F, Kakizaki S, Takashima Y, Seta Y. Delivery of siRNA to the brain using a combination of nose-to-brain delivery and cell-penetrating peptide-modified nano-micelles. Biomaterials. 2013;34(36):9220–6.
Simão Carlos MI, Zheng K, Garrett N, Arifin N, Workman DG, Kubajewska I, et al. Limiting the level of tertiary amines on polyamines leads to biocompatible nucleic acid vectors. Int J Pharm. 2017;526(1):106–24.
Li Y, Wang J, Lee CG, Wang CY, Gao SJ, Tang GP, et al. CNS gene transfer mediated by a novel controlled release system based on DNA complexes of degradable polycation PPE-EA: a comparison with polyethylenimine/DNA complexes. Gene Ther. 2004;11(1):109–14.
Alvarez-Maya I, Navarro-Quiroga I, Meraz-Ríos MA, Aceves J, Martinez-Fong D. In vivo gene transfer to dopamine neurons of rat substantia nigra via the high-affinity neurotensin receptor. Mol Med. 2001;7(3):186–92.
Alarcón-Arís D, Recasens A, Galofré M, Carballo-Carbajal I, Zacchi N, Ruiz-Bronchal E, et al. Selective α-Synuclein Knockdown in Monoamine Neurons by Intranasal Oligonucleotide Delivery: Potential Therapy for Parkinson’s Disease. Mol Ther. 2018;26(2):550–67.
Su Y, Sun B, Gao X, Dong X, Fu L, Zhang Y, et al. Intranasal Delivery of Targeted Nanoparticles Loaded With miR-132 to Brain for the Treatment of Neurodegenerative Diseases. Front Pharmacol. 2020;11:1165.
Wen MM. Olfactory targeting through intranasal delivery of biopharmaceutical drugs to the brain: current development. Discov Med. 2011;11(61):497–503.
Islam SU, Shehzad A, Ahmed MB, Lee YS. Intranasal Delivery of Nanoformulations: A Potential Way of Treatment for Neurological Disorders. Molecules (Basel, Switzerland). 2020;25(8):1929.
Lee D, Minko T. Nanotherapeutics for Nose-to-Brain Drug Delivery: An Approach to Bypass the Blood Brain Barrier. Pharmaceutics. 2021;13(12):2049.
Yadav S, Gandham SK, Panicucci R, Amiji MM. Intranasal brain delivery of cationic nanoemulsion-encapsulated TNFα siRNA in prevention of experimental neuroinflammation. Nanomedicine. 2016;12(4):987–1002.
Samaridou E, Walgrave H, Salta E, Álvarez DM, Castro-López V, Loza M, et al. Nose-to-brain delivery of enveloped RNA - cell permeating peptide nanocomplexes for the treatment of neurodegenerative diseases. Biomaterials. 2020;230:119657.
Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32(11):1959–72.
Gromnicova R, Davies HA, Sreekanthreddy P, Romero IA, Lund T, Roitt IM, et al. Glucose-Coated Gold Nanoparticles Transfer across Human Brain Endothelium and Enter Astrocytes In Vitro. PLoS One. 2013;8(12):e81043.
Pardridge WM. Blood-Brain Barrier and Delivery of Protein and Gene Therapeutics to Brain. Front Aging Neurosci. 2019;11:373.
Pardridge WM. Blood-brain barrier delivery of protein and non-viral gene therapeutics with molecular Trojan horses. J Control Release. 2007;122(3):345–8.
Kimura S, Harashima H. Current Status and Challenges Associated with CNS-Targeted Gene Delivery across the BBB. Pharmaceutics. 2020;12(12):1216.
Banks WA, Greig NH. Small molecules as central nervous system therapeutics: old challenges, new directions, and a philosophic divide. Future Med Chem. 2019;11(6):489–93.
Ko YT, Bhattacharya R, Bickel U. Liposome encapsulated polyethylenimine/ODN polyplexes for brain targeting. J Control Release. 2009;133(3):230–7.
da Cruz MT, Cardoso AL, de Almeida LP, Simões S, de Lima MC. Tf-lipoplex-mediated NGF gene transfer to the CNS: neuronal protection and recovery in an excitotoxic model of brain injury. Gene Ther. 2005;12(16):1242–52.
Xia C-F, Zhang Y, Zhang Y, Boado RJ, Pardridge WM. Intravenous siRNA of Brain Cancer with Receptor Targeting and Avidin-Biotin Technology. Pharm Res. 2007;24(12):2309–16.
Min HS, Kim HJ, Naito M, Ogura S, Toh K, Hayashi K, et al. Systemic Brain Delivery of Antisense Oligonucleotides across the Blood-Brain Barrier with a Glucose-Coated Polymeric Nanocarrier. Angew Chem Int Ed. 2020;59(21):8173–80.
An S, Kuang Y, Shen T, Li J, Ma H, Guo Y, et al. Brain-targeting delivery for RNAi neuroprotection against cerebral ischemia reperfusion injury. Biomaterials. 2013;34(35):8949–59.
Yuan Z, Zhao L, Zhang Y, Li S, Pan B, Hua L, et al. Inhibition of glioma growth by a GOLPH3 siRNA-loaded cationic liposomes. J Neurooncol. 2018;140(2):249–60.
Heidel JD, Yu Z, Liu JY, Rele SM, Liang Y, Zeidan RK, et al. Administration in non-human primates of escalating intravenous doses of targeted nanoparticles containing ribonucleotide reductase subunit M2 siRNA. Proc Natl Acad Sci USA. 2007;104(14):5715–21.
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–5.
Zhang R, Fu Y, Cheng M, Ma W, Zheng N, Wang Y, et al. sEVs(RVG) selectively delivers antiviral siRNA to fetus brain, inhibits ZIKV infection and mitigates ZIKV-induced microcephaly in mouse model. Mol Ther. 2022;30(5):2078–91.
Ren X, Zhao Y, Xue F, Zheng Y, Huang H, Wang W, et al. Exosomal DNA Aptamer Targeting α-Synuclein Aggregates Reduced Neuropathological Deficits in a Mouse Parkinson’s Disease Model. Molecular Therapy Nucleic Acids. 2019;17:726–40.
Cooper JM, Wiklander PB, Nordin JZ, Al-Shawi R, Wood MJ, Vithlani M, et al. Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov Disord. 2014;29(12):1476–85.
Banks WA, Sharma P, Bullock KM, Hansen KM, Ludwig N, Whiteside TL. Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation. International J Mol Sci. 2020;21(12):4407.
Chivet M, Javalet C, Laulagnier K, Blot B, Hemming FJ, Sadoul R. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles. 2014;3:24722-.
Fletcher AM, Kowalczyk TH, Padegimas L, Cooper MJ, Yurek DM. Transgene expression in the striatum following intracerebral injections of DNA nanoparticles encoding for human glial cell line-derived neurotrophic factor. Neuroscience. 2011;194:220–6.
Barbato G, Nisticò R, Triaca V. Exploiting Focused Ultrasound to Aid Intranasal Drug Delivery for Brain Therapy. Front Pharmacol. 2022;13:786475.
Lin J, Jo SB, Kim TH, Kim HW, Chew SY. RNA interference in glial cells for nerve injury treatment. J Tissue Eng. 2020;11:2041731420939224.
Song KH, Harvey BK, Borden MA. State-of-the-art of microbubble-assisted blood-brain barrier disruption. Theranostics. 2018;8(16):4393–408.
Meng Y, Hynynen K, Lipsman N. Applications of focused ultrasound in the brain: from thermoablation to drug delivery. Nat Rev Neurol. 2021;17(1):7–22.
Burgess A, Huang Y, Querbes W, Sah DW, Hynynen K. Focused ultrasound for targeted delivery of siRNA and efficient knockdown of Htt expression. J Control Release. 2012;163(2):125–9.
Zhao Z, Ukidve A, Kim J, Mitragotri S. Targeting Strategies for Tissue-Specific Drug Delivery. Cell. 2020;181(1):151–67.
Tschuch C, Schulz A, Pscherer A, Werft W, Benner A, Hotz-Wagenblatt A, et al. Off-target effects of siRNA specific for GFP. BMC Mol Biol. 2008;9:60.
Persengiev SP, Zhu X, Green MR. Nonspecific, concentration-dependent stimulation and repression of mammalian gene expression by small interfering RNAs (siRNAs). RNA (New York, NY). 2004;10(1):12–8.
Jackson AL, Linsley PS. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat Rev Drug Discovery. 2010;9(1):57–67.
Valori CF, Possenti A, Brambilla L, Rossi D. Challenges and Opportunities of Targeting Astrocytes to Halt Neurodegenerative Disorders. Cells. 2021;10(8):2019.
Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430(7000):631–9.
Tosi G, Pederzoli F, Belletti D, Vandelli MA, Forni F, Duskey JT, et al. Nanomedicine in Alzheimer’s disease: Amyloid beta targeting strategy. Prog Brain Res. 2019;245:57–88.
Gouras GK, Almeida CG, Takahashi RH. Intraneuronal Aβ accumulation and origin of plaques in Alzheimer’s disease. Neurobiol Aging. 2005;26(9):1235–44.
Ramachandran PS, Keiser MS, Davidson BL. Recent advances in RNA interference therapeutics for CNS diseases. Neurotherapeutics. 2013;10(3):473–85.
Gomes CV, Kaster MP, Tomé AR, Agostinho PM, Cunha RA. Adenosine receptors and brain diseases: Neuroprotection and neurodegeneration. Biochim Biophys Acta (BBA) Biomembr. 2011;1808(5):1380–99.
Li T, Quan Lan J, Fredholm BB, Simon RP, Boison D. Adenosine dysfunction in astrogliosis: cause for seizure generation? Neuron Glia Biol. 2007;3(4):353–66.
Cepeda C, Wu N, André VM, Cummings DM, Levine MS. The corticostriatal pathway in Huntington’s disease. Prog Neurobiol. 2007;81(5–6):253–71.
Shin J-Y, Fang Z-H, Yu Z-X, Wang C-E, Li S-H, Li X-J. Expression of mutant huntingtin in glial cells contributes to neuronal excitotoxicity. J Cell Biol. 2005;171(6):1001–12.
Acevedo-Torres K, Berríos L, Rosario N, Dufault V, Skatchkov S, Eaton MJ, et al. Mitochondrial DNA damage is a hallmark of chemically induced and the R6/2 transgenic model of Huntington’s disease. DNA Repair. 2009;8(1):126–36.
Iyengar S, Johnson KW, Ossipov MH, Aurora SK. CGRP and the Trigeminal System in Migraine. Headache. 2019;59(5):659–81.
Deen M, Correnti E, Kamm K, Kelderman T, Papetti L, Rubio-Beltrán E, et al. Blocking CGRP in migraine patients – a review of pros and cons. J Headache Pain. 2017;18(1):96.
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71(9):670–6.
Peeraully T. Multiple System Atrophy. Semin Neurol. 2014;34(02):174–81.
Ntetsika T, Papathoma P-E, Markaki I. Novel targeted therapies for Parkinson’s disease. Mol Med. 2021;27(1):17.
Newland B, Dunnett SB, Dowd E. Targeting delivery in Parkinson’s disease. Drug Discovery Today. 2016;21(8):1313–20.
Boudreau RL, Rodríguez-Lebrón E, Davidson BL. RNAi medicine for the brain: progresses and challenges. Hum Mol Genet. 2011;20(R1):R21–7.
Kraus-Perrotta C, Lagalwar S. Expansion, mosaicism and interruption: mechanisms of the CAG repeat mutation in spinocerebellar ataxia type 1. Cerebellum Ataxias. 2016;3(1):20.
Przedborski S, Vila M, Jackson-Lewis V. Neurodegeneration: what is it and where are we? J Clin Investig. 2003;111(1):3–10.
Budday S, Ovaert TC, Holzapfel GA, Steinmann P, Kuhl E. Fifty Shades of Brain: A Review on the Mechanical Testing and Modeling of Brain Tissue. Arch Comput Methods Eng. 2020;27(4):1187–230.
Pfisterer U, Khodosevich K. Neuronal survival in the brain: neuron type-specific mechanisms. Cell Death & Disease. 2017;8(3):e2643-e.
Dugger BN, Dickson DW. Pathology of Neurodegenerative Diseases. Cold Spring Harb Perspect Biol. 2017;9(7):a028035.
Gorman AM. Neuronal cell death in neurodegenerative diseases: recurring themes around protein handling. J Cell Mol Med. 2008;12(6a):2263–80.
Jin G-Z, Chakraborty A, Lee J-H, Knowles JC, Kim H-W. Targeting with nanoparticles for the therapeutic treatment of brain diseases. J Tissue Eng. 2020;11:2041731419897460.
Pennartz CMA, Dora S, Muckli L, Lorteije JAM. Towards a Unified View on Pathways and Functions of Neural Recurrent Processing. Trends Neurosci. 2019;42(9):589–603.
Slow EJ, Graham RK, Hayden MR. To be or not to be toxic: aggregations in Huntington and Alzheimer disease. Trends Genet TIG. 2006;22(8):408–11.
Miguel-Hidalgo JJ, Alvarez XA, Cacabelos R, Quack G. Neuroprotection by memantine against neurodegeneration induced by beta-amyloid(1–40). Brain Res. 2002;958(1):210–21.
Ikonomovic MD, Mizukami K, Warde D, Sheffield R, Hamilton R, Wenthold RJ, et al. Distribution of glutamate receptor subunit NMDAR1 in the hippocampus of normal elderly and patients with Alzheimer’s disease. Exp Neurol. 1999;160(1):194–204.
Cheyuo C, Aziz M, Wang P. Neurogenesis in Neurodegenerative Diseases: Role of MFG-E8. Front Neurosci. 2019;13:569.
Herculano-Houzel S. The human brain in numbers: a linearly scaled-up primate brain. Front Hum Neurosci. 2009;3:31.
Kwon EJ, Lasiene J, Jacobson BE, Park IK, Horner PJ, Pun SH. Targeted nonviral delivery vehicles to neural progenitor cells in the mouse subventricular zone. Biomaterials. 2010;31(8):2417–24.
Chu DS, Schellinger JG, Bocek MJ, Johnson RN, Pun SH. Optimization of Tet1 ligand density in HPMA-co-oligolysine copolymers for targeted neuronal gene delivery. Biomaterials. 2013;34(37):9632–7.
Garcia-Chica J, D Paraiso WK, Tanabe S, Serra D, Herrero L, Casals N, et al. An overview of nanomedicines for neuron targeting. Nanomedicine. 2020;15(16):1617–36.
Lafon M. Rabies virus receptors. J Neurovirol. 2005;11(1):82–7.
Kumar P, Wu H, McBride JL, Jung K-E, Hee Kim M, Davidson BL, et al. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448(7149):39–43.
Wang P, Zheng X, Guo Q, Yang P, Pang X, Qian K, et al. Systemic delivery of BACE1 siRNA through neuron-targeted nanocomplexes for treatment of Alzheimer’s disease. J Control Release. 2018;279:220–33.
Zheng X, Pang X, Yang P, Wan X, Wei Y, Guo Q, et al. A hybrid siRNA delivery complex for enhanced brain penetration and precise amyloid plaque targeting in Alzheimer’s disease mice. Acta Biomater. 2017;49:388–401.
Kim SS, Ye C, Kumar P, Chiu I, Subramanya S, Wu H, et al. Targeted delivery of siRNA to macrophages for anti-inflammatory treatment. Mol Ther. 2010;18(5):993–1001.
Tanaka H, Nakatani T, Furihata T, Tange K, Nakai Y, Yoshioka H, et al. In Vivo Introduction of mRNA Encapsulated in Lipid Nanoparticles to Brain Neuronal Cells and Astrocytes via Intracerebroventricular Administration. Mol Pharm. 2018;15(5):2060–7.
Zhang C, Gu Z, Shen L, Liu X, Lin H. In vivo Evaluation and Alzheimer’s Disease Treatment Outcome of siRNA Loaded Dual Targeting Drug Delivery System. Curr Pharm Biotechnol. 2019;20(1):56–62.
Kwon EJ, Skalak M, Lo BuR, Bhatia SN. Neuron-Targeted Nanoparticle for siRNA Delivery to Traumatic Brain Injuries. ACS Nano. 2016;10(8):7926–33.
Rungta RL, Choi HB, Lin PJ, Ko RW, Ashby D, Nair J, et al. Lipid Nanoparticle Delivery of siRNA to Silence Neuronal Gene Expression in the Brain. Mol Ther Nucleic Acids. 2013;2(12):e136.
Stevenson R, Samokhina E, Rossetti I, Morley JW, Buskila Y. Neuromodulation of Glial Function During Neurodegeneration. Front Cell Neurosci. 2020;14:278.
Aamodt S. Focus on glia and disease. Nat Neurosci. 2007;10(11):1349.
Kery R, Chen APF, Kirschen GW. Genetic targeting of astrocytes to combat neurodegenerative disease. Neural Regen Res. 2020;15(2):199–211.
Maragakis NJ, Rothstein JD. Glutamate Transporters in Neurologic Disease. Arch Neurol. 2001;58(3):365–70.
Neher JJ, Neniskyte U, Zhao J-W, Bal-Price A, Tolkovsky AM, Brown GC. Inhibition of Microglial Phagocytosis Is Sufficient To Prevent Inflammatory Neuronal Death. J Immunol. 2011;186(8):4973.
Glezer I, Simard AR, Rivest S. Neuroprotective role of the innate immune system by microglia. Neuroscience. 2007;147(4):867–83.
Zhao N, Francis NL, Calvelli HR, Moghe PV. Microglia-targeting nanotherapeutics for neurodegenerative diseases. APL Bioengineering. 2020;4(3):030902.
Gu X, Song Q, Zhang Q, Huang M, Zheng M, Chen J, et al. Clearance of two organic nanoparticles from the brain via the paravascular pathway. J Control Release. 2020;322:31–41.
Tang Y, Le W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol Neurobiol. 2016;53(2):1181–94.
Cherry JD, Olschowka JA, O’Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation. 2014;11:98.
Chen K, Iribarren P, Hu J, Chen J, Gong W, Cho EH, et al. Activation of Toll-like Receptor 2 on Microglia Promotes Cell Uptake of Alzheimer Disease-associated Amyloid β Peptide*. J Biol Chem. 2006;281(6):3651–9.
Deczkowska A, Keren-Shaul H, Weiner A, Colonna M, Schwartz M, Amit I. Disease-Associated Microglia: A Universal Immune Sensor of Neurodegeneration. Cell. 2018;173(5):1073–81.
Wang J, Wang J, Wang J, Yang B, Weng Q, He Q. Targeting Microglia and Macrophages: A Potential Treatment Strategy for Multiple Sclerosis. Front Pharmacol. 2019;10:286.
Li K, Li J, Zheng J, Qin S. Reactive Astrocytes in Neurodegenerative Diseases. Aging Dis. 2019;10(3):664–75.
Phatnani H, Maniatis T. Astrocytes in neurodegenerative disease. Cold Spring Harb Perspect Biol. 2015;7(6):a020628.
Izrael M, Slutsky SG, Revel M. Rising Stars: Astrocytes as a Therapeutic Target for ALS Disease. Front Neurosci. 2020;14:824.
Love S. Demyelinating diseases. J Clin Pathol. 2006;59(11):1151–9.
Georgiou E, Sidiropoulou K, Richter J, Papaneophytou C, Sargiannidou I, Kagiava A, et al. Gene therapy targeting oligodendrocytes provides therapeutic benefit in a leukodystrophy model. Brain. 2017;140(3):599–616.
Guo S, Cázarez-Márquez F, Jiao H, Foppen E, Korpel NL, Grootemaat AE, et al. Specific Silencing of Microglial Gene Expression in the Rat Brain by Nanoparticle-Based Small Interfering RNA Delivery. ACS Appl Mater Interfaces. 2022;14(4):5066–79.
Minami SS, Sun B, Popat K, Kauppinen T, Pleiss M, Zhou Y, et al. Selective targeting of microglia by quantum dots. J Neuroinflammation. 2012;9(1):22.
Vu TQ, Maddipati R, Blute TA, Nehilla BJ, Nusblat L, Desai TA. Peptide-conjugated quantum dots activate neuronal receptors and initiate downstream signaling of neurite growth. Nano Lett. 2005;5(4):603–7.
Gao W, Li J. Targeted siRNA delivery reduces nitric oxide mediated cell death after spinal cord injury. J Nanobiotechnology. 2017;15(1):38.
Maes ME, Colombo G, Schulz R, Siegert S. Targeting microglia with lentivirus and AAV: Recent advances and remaining challenges. Neurosci Lett. 2019;707:134310.
Delzor A, Escartin C, Déglon N. Lentiviral vectors: a powerful tool to target astrocytes in vivo. Curr Drug Targets. 2013;14(11):1336–46.
Furman JL, Sama DM, Gant JC, Beckett TL, Murphy MP, Bachstetter AD, et al. Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer’s disease. J Neurosci. 2012;32(46):16129–40.
Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27(1):59–65.
Samaranch L, Salegio EA, San Sebastian W, Kells AP, Foust KD, Bringas JR, et al. Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates. Hum Gene Ther. 2012;23(4):382–9.
Terashima T, Ogawa N, Nakae Y, Sato T, Katagi M, Okano J, et al. Gene Therapy for Neuropathic Pain through siRNA-IRF5 Gene Delivery with Homing Peptides to Microglia. Mol Ther Nucleic Acids. 2018;11:203–15.
Gu J, Al-Bayati K, Ho EA. Development of antibody-modified chitosan nanoparticles for the targeted delivery of siRNA across the blood-brain barrier as a strategy for inhibiting HIV replication in astrocytes. Drug Deliv Transl Res. 2017;7(4):497–506.
Akita H, Nakatani T, Kuroki K, Maenaka K, Tange K, Nakai Y, et al. Effect of hydrophobic scaffold on the cellular uptake and gene transfection activities of DNA-encapsulating liposomal nanoparticles via intracerebroventricular administration. Int J Pharm. 2015;490(1–2):142–5.
Rittchen S, Boyd A, Burns A, Park J, Fahmy TM, Metcalfe S, et al. Myelin repair in vivo is increased by targeting oligodendrocyte precursor cells with nanoparticles encapsulating leukaemia inhibitory factor (LIF). Biomaterials. 2015;56:78–85.
Querbes W, Ge P, Zhang W, Fan Y, Costigan J, Charisse K, et al. Direct CNS delivery of siRNA mediates robust silencing in oligodendrocytes. Oligonucleotides. 2009;19(1):23–9.
Chen Q, Butler D, Querbes W, Pandey RK, Ge P, Maier MA, et al. Lipophilic siRNAs mediate efficient gene silencing in oligodendrocytes with direct CNS delivery. J Control Release. 2010;144(2):227–32.
Gardlík R, Pálffy R, Hodosy J, Lukács J, Turna J, Celec P. Vectors and delivery systems in gene therapy. Med Sci Monit. 2005;11(4):Ra110–21.
Sharma D, Arora S, Singh J, Layek B. A review of the tortuous path of nonviral gene delivery and recent progress. Int J Biol Macromol. 2021;183:2055–73.
Khalil IA, Kogure K, Akita H, Harashima H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol Rev. 2006;58(1):32–45.
Buck J, Grossen P, Cullis PR, Huwyler J, Witzigmann D. Lipid-Based DNA Therapeutics: Hallmarks of Non-Viral Gene Delivery. ACS Nano. 2019;13(4):3754–82.
Wagenaar TR, Tolstykh T, Shi C, Jiang L, Zhang J, Li Z, et al. Identification of the endosomal sorting complex required for transport-I (ESCRT-I) as an important modulator of anti-miR uptake by cancer cells. Nucleic Acids Res. 2015;43(2):1204–15.
Crooke ST, Wang S, Vickers TA, Shen W, Liang XH. Cellular uptake and trafficking of antisense oligonucleotides. Nat Biotechnol. 2017;35(3):230–7.
Schlich M, Palomba R, Costabile G, Mizrahy S, Pannuzzo M, Peer D, et al. Cytosolic delivery of nucleic acids: The case of ionizable lipid nanoparticles. Bioeng Transl Med. 2021;6(2):e10213.
Taylor RE, Zahid M. Cell Penetrating Peptides, Novel Vectors for Gene Therapy. Pharmaceutics. 2020;12(3):225.
Somiya M, Liu Q, Kuroda S. Current Progress of Virus-mimicking Nanocarriers for Drug Delivery. Nanotheranostics. 2017;1(4):415–29.
Oehlke J, Scheller A, Wiesner B, Krause E, Beyermann M, Klauschenz E, et al. Cellular uptake of an alpha-helical amphipathic model peptide with the potential to deliver polar compounds into the cell interior non-endocytically. Biochem Biophys Acta. 1998;1414(1–2):127–39.
Li W, Nicol F, Szoka FC. GALA: a designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery. Adv Drug Deliv Rev. 2004;56(7):967–85.
Wyman TB, Nicol F, Zelphati O, Scaria PV, Plank C, Szoka FC Jr. Design, synthesis, and characterization of a cationic peptide that binds to nucleic acids and permeabilizes bilayers. Biochemistry. 1997;36(10):3008–17.
Melikov K, Chernomordik LV. Arginine-rich cell penetrating peptides: from endosomal uptake to nuclear delivery. Cell Mol Life Sci CMLS. 2005;62(23):2739–49.
Kichler A, Mason AJ, Bechinger B. Cationic amphipathic histidine-rich peptides for gene delivery. Biochem Biophys Acta. 2006;1758(3):301–7.
Sonawane ND, Szoka FC Jr, Verkman AS. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J Biol Chem. 2003;278(45):44826–31.
Medina-Kauwe LK, Xie J, Hamm-Alvarez S. Intracellular trafficking of nonviral vectors. Gene Ther. 2005;12(24):1734–51.
Xu Y, Szoka FC Jr. Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry. 1996;35(18):5616–23.
Schaffer DV, Fidelman NA, Dan N, Lauffenburger DA. Vector unpacking as a potential barrier for receptor-mediated polyplex gene delivery. Biotechnol Bioeng. 2000;67(5):598–606.
Lorenz C, Fotin-Mleczek M, Roth G, Becker C, Dam TC, Verdurmen WP, et al. Protein expression from exogenous mRNA: uptake by receptor-mediated endocytosis and trafficking via the lysosomal pathway. RNA Biol. 2011;8(4):627–36.
Vermeulen LMP, Brans T, De Smedt SC, Remaut K, Braeckmans K. Methodologies to investigate intracellular barriers for nucleic acid delivery in non-viral gene therapy. Nano Today. 2018;21:74–90.
Vaughan EE, DeGiulio JV, Dean DA. Intracellular trafficking of plasmids for gene therapy: mechanisms of cytoplasmic movement and nuclear import. Curr Gene Ther. 2006;6(6):671–81.
Mirzayans R, Aubin RA, Paterson MC. Differential expression and stability of foreign genes introduced into human fibroblasts by nuclear versus cytoplasmic microinjection. Mutat Res. 1992;281(2):115–22.
Sebestyén MG, Ludtke JJ, Bassik MC, Zhang G, Budker V, Lukhtanov EA, et al. DNA vector chemistry: The covalent attachment of signal peptides to plasmid DNA. Nat Biotechnol. 1998;16(1):80–5.
Mesika A, Grigoreva I, Zohar M, Reich Z. A regulated, NFkappaB-assisted import of plasmid DNA into mammalian cell nuclei. Mol Ther. 2001;3(5 Pt 1):653–7.
Vandenbroucke RE, Lucas B, Demeester J, De Smedt SC, Sanders NN. Nuclear accumulation of plasmid DNA can be enhanced by non-selective gating of the nuclear pore. Nucleic Acids Res. 2007;35(12):e86.
Park KM, Kang HC, Cho JK, Chung IJ, Cho SH, Bae YH, et al. All-trans-retinoic acid (ATRA)-grafted polymeric gene carriers for nuclear translocation and cell growth control. Biomaterials. 2009;30(13):2642–52.
Charoensit P, Kawakami S, Higuchi Y, Yamashita F, Hashida M. Enhanced growth inhibition of metastatic lung tumors by intravenous injection of ATRA-cationic liposome/IL-12 pDNA complexes in mice. Cancer Gene Ther. 2010;17(7):512–22.
Grandinetti G, Reineke TM. Exploring the Mechanism of Plasmid DNA Nuclear Internalization with Polymer-Based Vehicles. Mol Pharm. 2012;9(8):2256–67.
Grandinetti G, Smith AE, Reineke TM. Membrane and Nuclear Permeabilization by Polymeric pDNA Vehicles: Efficient Method for Gene Delivery or Mechanism of Cytotoxicity? Mol Pharm. 2012;9(3):523–38.
Durymanov MO, Yarutkin AV, Khramtsov YV, Rosenkranz AA, Sobolev AS. Live imaging of transgene expression in Cloudman S91 melanoma cells after polyplex-mediated gene delivery. J Control Release. 2015;215:73–81.
Kirchenbuechler I, Kirchenbuechler D, Elbaum M. Correlation between cationic lipid-based transfection and cell division. Exp Cell Res. 2016;345(1):1–5.
Durymanov M, Reineke J. Non-viral Delivery of Nucleic Acids: Insight Into Mechanisms of Overcoming Intracellular Barriers. Front Pharmacol. 2018;9:971.
Chowdhury EH. Nuclear targeting of viral and non-viral DNA. Expert Opin Drug Deliv. 2009;6(7):697–703.
Monzio Compagnoni G, Di Fonzo A, Corti S, Comi GP, Bresolin N, Masliah E. The Role of Mitochondria in Neurodegenerative Diseases: the Lesson from Alzheimer’s Disease and Parkinson’s Disease. Mol Neurobiol. 2020;57(7):2959–80.
Reddy PH. Role of mitochondria in neurodegenerative diseases: mitochondria as a therapeutic target in Alzheimer's disease. CNS Spectrums. 2009;14(8 Suppl 7):8–13; discussion 16–8.
Moreira PI, Zhu X, Wang X, Lee HG, Nunomura A, Petersen RB, et al. Mitochondria: a therapeutic target in neurodegeneration. Biochem Biophys Acta. 2010;1802(1):212–20.
Burchell VS, Gandhi S, Deas E, Wood NW, Abramov AY, Plun-Favreau H. Targeting mitochondrial dysfunction in neurodegenerative disease: Part I. Expert Opin Ther Targets. 2010;14(4):369–85.
Zhang Y, Yang H, Wei D, Zhang X, Wang J, Wu X, et al. Mitochondria-targeted nanoparticles in treatment of neurodegenerative diseases. Exploration. 2021;1(3):20210115.
Tabish TA, Hamblin MR. Mitochondria-targeted nanoparticles (mitoNANO): An emerging therapeutic shortcut for cancer. Biomater Biosyst. 2021;3:100023.
Lee J-M, Correia K, Loupe J, Kim K-H, Barker D, Hong EP, et al. CAG Repeat Not Polyglutamine Length Determines Timing of Huntington’s Disease Onset. Cell. 2019;178(4):887-900.e14.
Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 1995;18(12):527–35.
Picconi B, Passino E, Sgobio C, Bonsi P, Barone I, Ghiglieri V, et al. Plastic and behavioral abnormalities in experimental Huntington’s disease: a crucial role for cholinergic interneurons. Neurobiol Dis. 2006;22(1):143–52.
Dragunow M. Human Brain Neuropharmacology: A Platform for Translational Neuroscience. Trends Pharmacol Sci. 2020;41(11):777–92.
Barker RA, Björklund A. Animal Models of Parkinson’s Disease: Are They Useful or Not? J Parkinsons Dis. 2020;10:1335–42.
Mishra MK, Beaty CA, Lesniak WG, Kambhampati SP, Zhang F, Wilson MA, et al. Dendrimer brain uptake and targeted therapy for brain injury in a large animal model of hypothermic circulatory arrest. ACS Nano. 2014;8(3):2134–47.
Kannan S, Dai H, Navath RS, Balakrishnan B, Jyoti A, Janisse J, et al. Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci Transl Med. 2012;4(130):130ra46.
Zhang F, Mastorakos P, Mishra MK, Mangraviti A, Hwang L, Zhou J, et al. Uniform brain tumor distribution and tumor associated macrophage targeting of systemically administered dendrimers. Biomaterials. 2015;52:507–16.
Nance E, Porambo M, Zhang F, Mishra MK, Buelow M, Getzenberg R, et al. Systemic dendrimer-drug treatment of ischemia-induced neonatal white matter injury. J Control Release. 2015;214:112–20.
Zhang F, Lin Y-A, Kannan S, Kannan RM. Targeting specific cells in the brain with nanomedicines for CNS therapies. J Control Release. 2016;240:212–26.
Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol. 2010;6(7):393–403.
Acknowledgements
Our collaborative efforts and the development of CNS-specific delivery strategies for nucleic acid therapeutics are supported by grants from the Massachusetts Life Sciences Center and the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This invited manuscript is submitted for the thematic issue of the journal Pharmaceutical Research focusing on “Nucleic Acid Delivery” edited by Drs. Galen Shi and Zheng-Rong Lu.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
D’Souza, A., Nozohouri, S., Bleier, B.S. et al. CNS Delivery of Nucleic Acid Therapeutics: Beyond the Blood–Brain Barrier and Towards Specific Cellular Targeting. Pharm Res 40, 77–105 (2023). https://doi.org/10.1007/s11095-022-03433-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03433-5