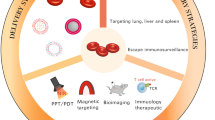
Abstract
In the past five decades, red blood cells (RBCs) have been extensively explored as drug delivery systems due to their distinguishing potential in modulating the pharmacokinetic, pharmacodynamics, and biological activity of carried payloads. The extensive interests in RBC-mediated drug delivery technologies are in part derived from RBCs’ unique biological features such as long circulation time, wide access to many tissues in the body, and low immunogenicity. Owing to these outstanding properties, a large body of efforts have led to the development of various RBC-inspired strategies to enable precise drug delivery with enhanced therapeutic efficacy and reduced off-target toxicity. In this review, we discuss emerging concepts and new advances in such RBC-inspired strategies, including native RBCs, ghost RBCs, RBC-mimetic nanoparticles, and RBC-derived extracellular vesicles, for drug delivery.






Similar content being viewed by others
References
Li W, Tang J, Lee D, Tice TR, Schwendeman SP, Prausnitz MR. Clinical translation of long-acting drug delivery formulations. Nature Reviews Materials. 2022.
Furtado D, Björnmalm M, Ayton S, Bush AI, Kempe K, Caruso F. Overcoming the Blood-Brain Barrier: The Role of Nanomaterials in Treating Neurological Diseases. Adv Mater. 2018;30(46):1801362.
Leandro K, Bicker J, Alves G, Falcão A, Fortuna A. ABC transporters in drug-resistant epilepsy: mechanisms of upregulation and therapeutic approaches. Pharmacol Res. 2019;144:357–76.
Dou Y, Li C, Li L, Guo J, Zhang J. Bioresponsive drug delivery systems for the treatment of inflammatory diseases. J Control Release. 2020;327:641–66.
Vargason AM, Anselmo AC, Mitragotri S. The evolution of commercial drug delivery technologies. Nature Biomedical Engineering. 2021;5(9):951–67.
Brown TD, Whitehead KA, Mitragotri S. Materials for oral delivery of proteins and peptides. Nat Rev Mater. 2020;5(2):127–48.
Holloway Julianne L. Drug delivery in stealth mode. Science Translational Medicine. 2018;10(471):eaaw0523.
Fenton OS, Olafson KN, Pillai PS, Mitchell MJ, Langer R. Advances in Biomaterials for Drug Delivery. Adv Mater. 2018;30(29):1705328.
Durán-Lobato M, Niu Z, Alonso MJ. Oral Delivery of Biologics for Precision Medicine. Adv Mater. 2020;32(13):1901935.
Zhao Z, Ukidve A, Kim J, Mitragotri S. Targeting Strategies for Tissue-Specific Drug Delivery. Cell. 2020;181(1):151–67.
Glassman PM, Hood ED, Ferguson LT, Zhao Z, Siegel DL, Mitragotri S, Brenner JS, Muzykantov VR. Red blood cells: The metamorphosis of a neglected carrier into the natural mothership for artificial nanocarriers. Adv Drug Deliv Rev. 2021;178: 113992.
Salunkhe SA, Chitkara D, Mahato RI, Mittal A. Lipid based nanocarriers for effective drug delivery and treatment of diabetes associated liver fibrosis. Adv Drug Deliv Rev. 2021;173:394–415.
George-Gay B, Parker K. Understanding the complete blood count with differential. J Perianesth Nurs. 2003;18(2):96–117.
Han X, Wang C, Liu Z. Red Blood Cells as Smart Delivery Systems. Bioconjug Chem. 2018;29(4):852–60.
Mantel P-Y, Hjelmqvist D, Walch M, Kharoubi-Hess S, Nilsson S, Ravel D, Ribeiro M, Grüring C, Ma S, Padmanabhan P, Trachtenberg A, Ankarklev J, Brancucci NM, Huttenhower C, Duraisingh MT, Ghiran I, Kuo WP, Filgueira L, Martinelli R, Marti M. Infected erythrocyte-derived extracellular vesicles alter vascular function via regulatory Ago2-miRNA complexes in malaria. Nat Commun. 2016;7(1):12727.
Zhu R, Avsievich T, Popov A, Bykov A, Meglinski I. In vivo nano-biosensing element of red blood cell-mediated delivery. Biosens Bioelectron. 2021;175: 112845.
Zhang X, Luo M, Dastagir SR, Nixon M, Khamhoung A, Schmidt A, Lee A, Subbiah N, McLaughlin DC, Moore CL, Gribble M, Bayhi N, Amin V, Pepi R, Pawar S, Lyford TJ, Soman V, Mellen J, Carpenter CL, Turka LA, Wickham TJ, Chen TF. Engineered red blood cells as an off-the-shelf allogeneic anti-tumor therapeutic. Nat Commun. 2021;12(1):2637.
Jiang Q, Luo Z, Men Y, Yang P, Peng H, Guo R, Tian Y, Pang Z, Yang W. Red blood cell membrane-camouflaged melanin nanoparticles for enhanced photothermal therapy. Biomaterials. 2017;143:29–45.
Brenner JS, Mitragotri S, Muzykantov VR. Red Blood Cell Hitchhiking: A Novel Approach for Vascular Delivery of Nanocarriers. Annu Rev Biomed Eng. 2021;23(1):225–48.
Hu C-MJ, Fang RH, Zhang L. Erythrocyte-Inspired Delivery Systems. Advanced Healthcare Materials. 2012;1(5):537–547.
Oldenborg P-A, Zheleznyak A, Fang Y-F, Lagenaur Carl F, Gresham Hattie D, Lindberg FP. Role of CD47 as a Marker of Self on Red Blood Cells. Science. 2000;288(5473):2051–4.
Izzati Mat Rani NN, Alzubaidi ZM, Azhari H, Mustapa F, Iqbal Mohd Amin MC. Novel engineering: Biomimicking erythrocyte as a revolutionary platform for drugs and vaccines delivery. European Journal of Pharmacology. 2021;900:174009.
Sun Y, Su J, Liu G, Chen J, Zhang X, Zhang R, Jiang M, Qiu M. Advances of blood cell-based drug delivery systems. Eur J Pharm Sci. 2017;96:115–28.
Luk BT, Zhang L. Cell membrane-camouflaged nanoparticles for drug delivery. J Control Release. 2015;220:600–7.
Villa CH, Anselmo AC, Mitragotri S, Muzykantov V. Red blood cells: Supercarriers for drugs, biologicals, and nanoparticles and inspiration for advanced delivery systems. Adv Drug Deliv Rev. 2016;106:88–103.
Sternberg N, Georgieva R Fau - Duft K, Duft K Fau - Bäumler H, Bäumler H. Surface-modified loaded human red blood cells for targeting and delivery of drugs. (1464–5246 (Electronic)).
Xie J, Shen Q, Huang K, Zheng T, Cheng L, Zhang Z, Yu Y, Liao G, Wang X, Li C. Oriented Assembly of Cell-Mimicking Nanoparticles via a Molecular Affinity Strategy for Targeted Drug Delivery. ACS Nano. 2019;13(5):5268–77.
Glassman PM, Villa CH, Ukidve A, Zhao Z, Smith P, Mitragotri S, Russell AJ, Brenner JS, Muzykantov VR. Vascular Drug Delivery Using Carrier Red Blood Cells: Focus on RBC Surface Loading and Pharmacokinetics. Pharmaceutics. 2020;12(5).
Gheibi Hayat SM, Bianconi V, Pirro M, Sahebkar A. Stealth functionalization of biomaterials and nanoparticles by CD47 mimicry. Int J Pharm. 2019;569: 118628.
Rao L, Bu L-L, Xu J-H, Cai B, Yu G-T, Yu X, He Z, Huang Q, Li A, Guo S-S, Zhang W-F, Liu W, Sun Z-J, Wang H, Wang T-H, Zhao X-Z. Red Blood Cell Membrane as a Biomimetic Nanocoating for Prolonged Circulation Time and Reduced Accelerated Blood Clearance. Small. 2015;11(46):6225–36.
Podsiedlik M, Markowicz-Piasecka M, Sikora J. Erythrocytes as model cells for biocompatibility assessment, cytotoxicity screening of xenobiotics and drug delivery. Chem Biol Interact. 2020;332: 109305.
Wadhwa R, Aggarwal T, Thapliyal N, Kumar A, Priya, Yadav P, Kumari V, Reddy BSC, Chandra P, Maurya PK. Red blood cells as an efficient in vitro model for evaluating the efficacy of metallic nanoparticles. 3 Biotech. 2019;9(7):279.
Pierigè F, Bigini N, Rossi L, Magnani MJWIRN, Nanobiotechnology. Reengineering red blood cells for cellular therapeutics and diagnostics. 2017;9(5):e1454.
Malhotra S, Dumoga S, Sirohi P, Singh N. Red Blood Cells-Derived Vesicles for Delivery of Lipophilic Drug Camptothecin. ACS Appl Mater Interfaces. 2019;11(25):22141–51.
Rossi L, Serafini S, Cenerini L, Picardi F, Bigi L, Panzani I, Magnani M. Erythrocyte-mediated delivery of dexamethasone in patients with chronic obstructive pulmonary disease. Biotechnol Appl Biochem. 2001;33(2):85–9.
Su J, Sun H, Meng Q, Yin Q, Zhang P, Zhang Z, Yu H, Li Y. Bioinspired Nanoparticles with NIR-Controlled Drug Release for Synergetic Chemophotothermal Therapy of Metastatic Breast Cancer. Adv Func Mater. 2016;26(41):7495–506.
Aryal S, Nguyen TDT, Pitchaimani A, Shrestha TB, Biller D, Troyer D. Membrane Fusion-Mediated Gold Nanoplating of Red Blood Cell: A Bioengineered CT-Contrast Agent. ACS Biomater Sci Eng. 2017;3(1):36–41.
Chen JL, Dhanaliwala AH, Dixon AJ, Farry JM, Hossack JA, Klibanov AL. Acoustically active red blood cell carriers for ultrasound-triggered drug delivery with photoacoustic tracking. In.2015 IEEE International Ultrasonics Symposium (IUS): IEEE; 2015. p. 1–4.
Dixon A, Farry J, Chen J, Dhanaliwala AH, Hossack JA, Klibanov A. Photoacoustic imaging of stimuli-responsive red blood cell drug delivery agents. In.2016 IEEE International Ultrasonics Symposium (IUS); 2016. p. 1–4.
Bax B, Bain M, Fairbanks L, Webster A, Chalmers RJBjoh. In vitro and in vivo studies with human carrier erythrocytes loaded with polyethylene glycol‐conjugated and native adenosine deaminase. 2000;109(3):549-554.
Liu W-L, Liu T, Zou M-Z, Yu W-Y, Li C-X, He Z-Y, Zhang M-K, Liu M-D, Li Z-H, Feng J, Zhang X-Z. Aggressive Man-Made Red Blood Cells for Hypoxia-Resistant Photodynamic Therapy. Adv Mater. 2018;30(35):1802006.
Domenech C, Thomas X, Chabaud S, Baruchel A, Gueyffier F, Mazingue F, Auvrignon A, Corm S, Dombret H, Chevallier P, Galambrun C, Huguet F, Legrand F, Mechinaud F, Vey N, Philip I, Liens D, Godfrin Y, Rigal D, Bertrand Y. l-asparaginase loaded red blood cells in refractory or relapsing acute lymphoblastic leukaemia in children and adults: results of the GRASPALL 2005–01 randomized trial. Br J Haematol. 2011;153(1):58–65.
Bodewes SB, van Leeuwen OB, Thorne AM, Lascaris B, Ubbink R, Lisman T, Monbaliu D, De Meijer VE, Nijsten MWN, Porte RJ. Oxygen Transport during Ex Situ Machine Perfusion of Donor Livers Using Red Blood Cells or Artificial Oxygen Carriers. International Journal of Molecular Sciences. 2021;22(1).
Dominici S, Laguardia ME, Serafini G, Chiarantini L, Fortini C, Tripiciano A, Brocca-Cofano E, Scoglio A, Caputo A, Fiorelli V, Gavioli R, Cafaro A, Ensoli B, Magnani M. Red blood cell-mediated delivery of recombinant HIV-1 Tat protein in mice induces anti-Tat neutralizing antibodies and CTL. Vaccine. 2003;21(17):2073–81.
Chiarantini L, Argnani R, Zucchini S, Stevanato L, Zabardi P, Grossi MP, Magnani M, Manservigi R. Red blood cells as delivery system for recombinant HSV-1 glycoprotein B: immunogenicity and protection in mice. Vaccine. 1997;15(3):276–80.
Zheng J, Lu C, Ding Y, Zhang J, Tan F, Liu J, Yang G, Wang Y, Li Z, Yang M, Yang Y, Gong W, Gao C. Red blood cell-hitchhiking mediated pulmonary delivery of ivermectin: Effects of nanoparticle properties. Int J Pharm. 2022;619: 121719.
Li C, Wang X, Li R, Yang X, Zhong Z, Dai Y, Fan Q, Lin Y, Zhang R, Liang T, Ye Y, Zhou M. Resveratrol-loaded PLGA nanoparticles functionalized with red blood cell membranes as a biomimetic delivery system for prolonged circulation time. Journal of Drug Delivery Science and Technology. 2019;54: 101369.
Chen Z-A, Wu S-H, Chen P, Chen Y-P, Mou C-Y. Critical Features for Mesoporous Silica Nanoparticles Encapsulated into Erythrocytes. ACS Appl Mater Interfaces. 2019;11(5):4790–8.
Raposo CJ, Cserny JD, Serena G, Chow JN, Cho P, Liu H, Kotler D, Sharei A, Bernstein H, John S. Engineered RBCs Encapsulating Antigen Induce Multi-Modal Antigen-Specific Tolerance and Protect Against Type 1 Diabetes. 2022;13.
Cremel M, Guérin N Fau - Horand F, Horand F Fau - Banz A, Banz A Fau - Godfrin Y, Godfrin Y. Red blood cells as innovative antigen carrier to induce specific immune tolerance. (1873–3476 (Electronic)).
Shi J, Kundrat L, Pishesha N, Bilate A, Theile C, Maruyama T, Dougan Stephanie K, Ploegh Hidde L, Lodish HF. Engineered red blood cells as carriers for systemic delivery of a wide array of functional probes. Proc Natl Acad Sci. 2014;111(28):10131–6.
Xia D, He H, Wang Y, Wang K, Zuo H, Gu H, Xu P, Hu Y. Ultrafast glucose-responsive, high loading capacity erythrocyte to self-regulate the release of insulin. Acta Biomater. 2018;69:301–12.
Wang C, Huang J, Zhang Y, Jia H, Chen B. Construction and evaluation of red blood cells-based drug delivery system for chemo-photothermal therapy. Colloids Surf, B. 2021;204: 111789.
Bachet J-B, Gay F, Maréchal R, Galais M-P, Adenis A, David Salako M, Cros J, Demetter P, Svrcek M, Bardier-Dupas AJP. Asparagine synthetase expression and phase I study with L-asparaginase encapsulated in red blood cells in patients with pancreatic adenocarcinoma. 2015;44(7):1141-1147.
Harisa GI, Badran MM, AlQahtani SA, Alanazi FK, Attia SM. Pravastatin chitosan nanogels-loaded erythrocytes as a new delivery strategy for targeting liver cancer. Saudi Pharmaceutical Journal. 2016;24(1):74–81.
Hammel P, Fabienne P, Mineur L, Metges J-P, Andre T, De La Fouchardiere C, Louvet C, El Hajbi F, Faroux R, Guimbaud R, Tougeron D, Bouche O, Lecomte T, Rebischung C, Tournigand C, Cros J, Kay R, Hamm A, Gupta A, Bachet J-B, El Hariry I. Erythrocyte-encapsulated asparaginase (eryaspase) combined with chemotherapy in second-line treatment of advanced pancreatic cancer: An open-label, randomized Phase IIb trial. Eur J Cancer. 2020;124:91–101.
Huang N-J, Pishesha N, Mukherjee J, Zhang S, Deshycka R, Sudaryo V, Dong M, Shoemaker CB, Lodish HF. Genetically engineered red cells expressing single domain camelid antibodies confer long-term protection against botulinum neurotoxin. Nat Commun. 2017;8(1):423.
Kosenko EA, Venediktova NI, Kudryavtsev AA, Ataullakhanov FI, Kaminsky YG, Felipo V, Montoliu C. Encapsulation of glutamine synthetase in mouse erythrocytes: a new procedure for ammonia detoxification. Biochem Cell Biol. 2008;86(6):469–76.
Hamidi M, Rafiei P, Azadi A, Mohammadi-Samani S. Encapsulation of Valproate-Loaded Hydrogel Nanoparticles in Intact Human Erythrocytes: A Novel Nano-cell Composite for Drug Delivery. J Pharm Sci. 2011;100(5):1702–11.
Hamidi M, Azimi K, Mohammadi-Samani SJJoP, Sciences P. Co-encapsulation of a drug with a protein in erythrocytes for improved drug loading and release: phenytoin and bovine serum albumin (BSA). 2011;14(1):46-59.
Yew NS, Dufour E, Przybylska M, Putelat J, Crawley C, Foster M, Gentry S, Reczek D, Kloss A, Meyzaud A, Horand F, Cheng SH, Godfrin Y. Erythrocytes encapsulated with phenylalanine hydroxylase exhibit improved pharmacokinetics and lowered plasma phenylalanine levels in normal mice. Mol Genet Metab. 2013;109(4):339–44.
Rossi L, Pierigè F, Carducci C, Gabucci C, Pascucci T, Canonico B, Bell SM, Fitzpatrick PA, Leuzzi V, Magnani M. Erythrocyte-mediated delivery of phenylalanine ammonia lyase for the treatment of phenylketonuria in BTBR-Pahenu2 mice. J Control Release. 2014;194:37–44.
Sarkissian Christineh N, Gámez A, Wang L, Charbonneau M, Fitzpatrick P, Lemontt Jeffrey F, Zhao B, Vellard M, Bell Sean M, Henschell C, Lambert A, Tsuruda L, Stevens Raymond C, Scriver CR. Preclinical evaluation of multiple species of PEGylated recombinant phenylalanine ammonia lyase for the treatment of phenylketonuria. Proc Natl Acad Sci. 2008;105(52):20894–9.
Bourgeaux V, Hequet O, Campion Y, Delcambre G, Chevrier A-M, Rigal D, Godfrin Y. Inositol hexaphosphate–loaded red blood cells prevent in vitro sickling. Transfusion. 2010;50(10):2176–84.
Lamarre Y, Bourgeaux V, Pichon A, Hardeman MR, Campion Y, Hardeman-Zijp M, Martin C, Richalet J-P, Bernaudin F, Driss F, Godfrin Y, Connes P. Effect of inositol hexaphosphate–loaded red blood cells (RBCs) on the rheology of sickle RBCs. Transfusion. 2013;53(3):627–36.
Muthuvel A, Rajamani R, Manikandan S, Sheeladevi R. Detoxification of formate by formate dehydrogenase-loaded erythrocytes and carbicarb in folate-deficient methanol-intoxicated rats. Clin Chim Acta. 2006;367(1):162–9.
Alexandrovich YG, Kosenko EA, Sinauridze EI, Obydennyi SI, Kireev II, Ataullakhanov FI, Kaminsky YG. Rapid Elimination of Blood Alcohol Using Erythrocytes: Mathematical Modeling and In Vitro Study. Biomed Res Int. 2017;2017:5849593.
Hamarat Baysal Ş, Uslan AH. Encapsulation of catalase and peg-catalase in erythrocyte. Artificial Cells, Blood Substitutes, and Biotechnology. 2001;29(5):359–66.
Briones E, Colino CI, Lanao JMJIjop. Study of the factors influencing the encapsulation of zidovudine in rat erythrocytes. 2010;401(1-2):41-46.
Harisa GI, Ibrahim MF, Alanazi FK. Erythrocyte-mediated delivery of pravastatin: In Vitro study of effect of hypotonic lysis on biochemical parameters and loading efficiency. Archives of Pharmacal Research. 2012;35(8):1431–1439.
Rossi L, Pierigè F, Antonelli A, Bigini N, Gabucci C, Peiretti E, Magnani M. Engineering erythrocytes for the modulation of drugs’ and contrasting agents’ pharmacokinetics and biodistribution. Adv Drug Deliv Rev. 2016;106:73–87.
Wang S, Chen R. pH-Responsive, Lysine-Based, Hyperbranched Polymers Mimicking Endosomolytic Cell-Penetrating Peptides for Efficient Intracellular Delivery. Chem Mater. 2017;29(14):5806–15.
Wang LL, Janes ME, Kumbhojkar N, Kapate N, Clegg JR, Prakash S, Heavey MK, Zhao Z, Anselmo AC, Mitragotri S. Cell therapies in the clinic. Bioeng Transl Med. 2021;6(2): e10214.
Bachet JB, Gay F Fau - Maréchal R, Maréchal R Fau - Galais M-P, Galais Mp Fau - Adenis A, Adenis A Fau - MsC DS, MsC Ds Fau - Cros J, Cros J Fau - Demetter P, Demetter P Fau - Svrcek M, Svrcek M Fau - Bardier-Dupas A, Bardier-Dupas A Fau - Emile J-F, Emile Jf Fau - Hammel P, Hammel P Fau - Ebenezer C, Ebenezer C Fau - Berlier W, Berlier W Fau - Godfrin Y, Godfrin Y Fau - André T, André T. Asparagine Synthetase Expression and Phase I Study With L-Asparaginase Encapsulated in Red Blood Cells in Patients With Pancreatic Adenocarcinoma. (1536–4828 (Electronic)).
Kontos S, Kourtis Iraklis C, Dane Karen Y, Hubbell JA. Engineering antigens for in situ erythrocyte binding induces T-cell deletion. Proc Natl Acad Sci. 2013;110(1):E60–8.
Zhao Y, Fan M, Chen Y, Liu Z, Shao C, Jin B, Wang X, Hui L, Wang S, Liao Z, Ling D, Tang R, Wang B. Surface-anchored framework for generating RhD-epitope stealth red blood cells. Science Advances;6(12):eaaw9679.
Wang C, Sun X, Cheng L, Yin S, Yang G, Li Y, Liu Z. Multifunctional Theranostic Red Blood Cells For Magnetic-Field-Enhanced in vivo Combination Therapy of Cancer. Adv Mater. 2014;26(28):4794–802.
Ferrari G, Thrasher AJ, Aiuti A. Gene therapy using haematopoietic stem and progenitor cells. Nat Rev Genet. 2021;22(4):216–34.
Pishesha N, Bilate Angelina M, Wibowo Marsha C, Huang N-J, Li Z, Deshycka R, Bousbaine D, Li H, Patterson Heide C, Dougan Stephanie K, Maruyama T, Lodish Harvey F, Ploegh HL. Engineered erythrocytes covalently linked to antigenic peptides can protect against autoimmune disease. Proc Natl Acad Sci. 2017;114(12):3157–62.
Bourgeaux V, Lanao JM, Bax BE, Godfrin Y. Drug-loaded erythrocytes: on the road toward marketing approval. (1177–8881 (Electronic)).
Kitao T Fau - Hattori K, Hattori K Fau - Takeshita M, Takeshita M. Agglutination of leukemic cells and daunomycin entrapped erythrocytes with lectin in vitro and in vivo. (0014–4754 (Print)).
Paulitschke M, Nash GB, Anstee DJ, Tanner MJ, Gratzer WB. Perturbation of red blood cell membrane rigidity by extracellular ligands. Blood. 1995;86(1):342–8.
Sharei A, Zoldan J, Adamo A, Sim Woo Y, Cho N, Jackson E, Mao S, Schneider S, Han M-J, Lytton-Jean A, Basto Pamela A, Jhunjhunwala S, Lee J, Heller Daniel A, Kang Jeon W, Hartoularos George C, Kim K-S, Anderson Daniel G, Langer R, Jensen KF. A vector-free microfluidic platform for intracellular delivery. Proc Natl Acad Sci. 2013;110(6):2082–7.
Lee J, Sharei A, Sim WY, Adamo A, Langer R, Jensen KF, Bawendi MG. Nonendocytic delivery of functional engineered nanoparticles into the cytoplasm of live cells using a novel. High-Throughput Microfluidic Device Nano Letters. 2012;12(12):6322–7.
Ding X, Stewart MP, Sharei A, Weaver JC, Langer RS, Jensen KF. High-throughput nuclear delivery and rapid expression of DNA via mechanical and electrical cell-membrane disruption. Nature Biomedical Engineering. 2017;1(3):0039.
Stewart MP, Sharei A, Ding X, Sahay G, Langer R, Jensen KF. In vitro and ex vivo strategies for intracellular delivery. Nature. 2016;538(7624):183–92.
Marx V. Cell biology: delivering tough cargo into cells. Nat Methods. 2016;13(1):37–40.
Szeto GL, Van Egeren D, Worku H, Sharei A, Alejandro B, Park C, Frew K, Brefo M, Mao S, Heimann M, Langer R, Jensen K, Irvine DJ. Microfluidic squeezing for intracellular antigen loading in polyclonal B-cells as cellular vaccines. Sci Rep. 2015;5(1):10276.
Prakash S, Kumbhojkar N, Clegg JR, Mitragotri S. Cell-bound nanoparticles for tissue targeting and immunotherapy: Engineering of the particle–membrane interface. Curr Opin Colloid Interface Sci. 2021;52: 101408.
Bush LM, Healy CP, Javdan SB, Emmons JC, Deans TL. Biological cells as therapeutic delivery vehicles. Trends Pharmacol Sci. 2021;42(2):106–18.
Wang Y, Pisapati AV, Zhang XF, Cheng X. Recent developments in nanomaterial-based shear-sensitive drug delivery systems. Adv Healthcare Mater. 2021;10(13):2002196.
Ferguson LT, Hood ED, Shuvaeva T, Shuvaev VV, Basil MC, Wang Z, Nong J, Ma X, Wu J, Myerson JW, Marcos-Contreras OA, Katzen J, Carl JM, Morrisey EE, Cantu E, Villa CH, Mitragotri S, Muzykantov VR, Brenner JS. Dual Affinity to RBCs and Target Cells (DART) Enhances Both Organ- and Cell Type-Targeting of Intravascular Nanocarriers. ACS Nano. 2022;16(3):4666–83.
Zou Y, Liu Y, Yang Z, Zhang D, Lu Y, Zheng M, Xue X, Geng J, Chung R, Shi B. Effective and targeted human orthotopic glioblastoma xenograft therapy via a multifunctional biomimetic nanomedicine. Adv Mater. 2018;30(51):1803717.
Wang P, Li X, Yao C, Wang W, Zhao M, El-Toni AM, Zhang F. Orthogonal near-infrared upconversion co-regulated site-specific O2 delivery and photodynamic therapy for hypoxia tumor by using red blood cell microcarriers. Biomaterials. 2017;125:90–100.
Zhu D-M, Wu L, Suo M, Gao S, Xie W, Zan M-H, Liu A, Chen B, Wu W-T, Ji L-W, Chen L-b, Huang H-M, Guo S-S, Zhang W-F, Zhao X-Z, Sun Z-J, Liu W. Engineered red blood cells for capturing circulating tumor cells with high performance. Nanoscale. 2018;10(13):6014–6023.
Mai TD, d’Orlyé F, Ménager C, Varenne A, Siaugue J-M. Red blood cells decorated with functionalized core–shell magnetic nanoparticles: elucidation of the adsorption mechanism. Chem Commun. 2013;49(47):5393–5.
Zhang K, Cao Y, Kuang Y, Liu M, Chen Y, Wang Z, Hong S, Wang J, Pei R. Gd2O3 and GH combined with red blood cells to improve the sensitivity of contrast agents for cancer targeting MR imaging. Biomaterials Science. 2017;5(1):46–9.
Cinti C, Taranta M, Naldi I, Grimaldi S. Newly Engineered Magnetic Erythrocytes for Sustained and Targeted Delivery of Anti-Cancer Therapeutic Compounds. PLoS ONE. 2011;6(2): e17132.
Wu Z, Li T, Li J, Gao W, Xu T, Christianson C, Gao W, Galarnyk M, He Q, Zhang L, Wang J. Turning Erythrocytes into Functional Micromotors. ACS Nano. 2014;8(12):12041–8.
Jain SK, Vyas SP. Magnetically responsive diclofenac sodium-loaded erythrocytes: Preparation and in vitro characterization. J Microencapsul. 1994;11(2):141–51.
Delcea M, Sternberg N, Yashchenok AM, Georgieva R, Bäumler H, Möhwald H, Skirtach AG. Nanoplasmonics for Dual-Molecule Release through Nanopores in the Membrane of Red Blood Cells. ACS Nano. 2012;6(5):4169–80.
Gao M, Hu A, Sun X, Wang C, Dong Z, Feng L, Liu Z. Photosensitizer decorated red blood cells as an ultrasensitive light-responsive drug delivery system. ACS Appl Mater Interfaces. 2017;9(7):5855–63.
Zhu Y-X, Jia H-R, Guo Y, Liu X, Zhou N, Liu P, Wu F-G. Repurposing Erythrocytes as a “Photoactivatable Bomb”: A General Strategy for Site-Specific Drug Release in Blood Vessels. Small. 2021;17(34):2100753.
Zhao Z, Ukidve A, Krishnan V, Fehnel A, Pan DC, Gao Y, Kim J, Evans MA, Mandal A, Guo J, Muzykantov VR, Mitragotri S. Systemic tumour suppression via the preferential accumulation of erythrocyte-anchored chemokine-encapsulating nanoparticles in lung metastases. Nature Biomedical Engineering. 2021;5(5):441–54.
Brenner JS, Pan DC, Myerson JW, Marcos-Contreras OA, Villa CH, Patel P, Hekierski H, Chatterjee S, Tao J-Q, Parhiz H, Bhamidipati K, Uhler TG, Hood ED, Kiseleva RY, Shuvaev VS, Shuvaeva T, Khoshnejad M, Johnston I, Gregory JV, Lahann J, Wang T, Cantu E, Armstead WM, Mitragotri S, Muzykantov V. Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat Commun. 2018;9(1):2684.
Zhao Z, Ukidve A, Gao Y, Kim J, Mitragotri S. Erythrocyte leveraged chemotherapy (ELeCt): Nanoparticle assembly on erythrocyte surface to combat lung metastasis. Science Advances;5(11):eaax9250.
Anselmo AC, Gupta V Fau - Zern BJ, Zern Bj Fau - Pan D, Pan D Fau - Zakrewsky M, Zakrewsky M Fau - Muzykantov V, Muzykantov V Fau - Mitragotri S, Mitragotri S. Delivering nanoparticles to lungs while avoiding liver and spleen through adsorption on red blood cells. (1936–086X (Electronic)).
Nikitin MP, Zelepukin IV, Shipunova VO, Sokolov IL, Deyev SM, Nikitin PI. Enhancement of the blood-circulation time and performance of nanomedicines via the forced clearance of erythrocytes. Nature Biomedical Engineering. 2020;4(7):717–31.
Chambers E, Mitragotri S. Long circulating nanoparticles via adhesion on red blood cells: Mechanism and extended circulation. Exp Biol Med. 2007;232(7):958–66.
Anselmo AC, Kumar S, Gupta V, Pearce AM, Ragusa A, Muzykantov V, Mitragotri S. Exploiting shape, cellular-hitchhiking and antibodies to target nanoparticles to lung endothelium: Synergy between physical, chemical and biological approaches. Biomaterials. 2015;68:1–8.
Zhang CY, Lin W, Gao J, Shi X, Davaritouchaee M, Nielsen AE, Mancini RJ, Wang Z. pH-Responsive Nanoparticles Targeted to Lungs for Improved Therapy of Acute Lung Inflammation/Injury. ACS Appl Mater Interfaces. 2019;11(18):16380–90.
Ding Y, Lv B, Zheng J, Lu C, Liu J, Lei Y, Yang M, Wang Y, Li Z, Yang Y, Gong W, Han J, Gao C. RBC-hitchhiking chitosan nanoparticles loading methylprednisolone for lung-targeting delivery. J Control Release. 2022;341:702–15.
Ye H, Shen Z, Wei M, Li Y. Red blood cell hitchhiking enhances the accumulation of nano- and micro-particles in the constriction of a stenosed microvessel. Soft Matter. 2021;17(1):40–56.
Aghili ZS, Mirzaei SA, Banitalebi-Dehkordi M. A potential hypothesis for 2019-nCoV infection therapy through delivery of recombinant ACE2 by red blood cell-hitchhiking. Journal of Biological Research-Thessaloniki. 2020;27(1):17.
Ukidve A, Zhao Z, Fehnel A, Krishnan V, Pan Daniel C, Gao Y, Mandal A, Muzykantov V, Mitragotri S. Erythrocyte-driven immunization via biomimicry of their natural antigen-presenting function. Proc Natl Acad Sci. 2020;117(30):17727–36.
Anderson HL, Brodsky IE, Mangalmurti NS. The Evolving Erythrocyte: Red Blood Cells as Modulators of Innate Immunity. J Immunol. 2018;201(5):1343.
Nombela I, Ortega-Villaizan MdM. Nucleated red blood cells: Immune cell mediators of the antiviral response. PLOS Pathogens. 2018;14(4):e1006910.
Chapanian R, Constantinescu I, Brooks DE, Scott MD, Kizhakkedathu JN. In vivo circulation, clearance, and biodistribution of polyglycerol grafted functional red blood cells. Biomaterials. 2012;33(10):3047–57.
Briones E, Colino CI, Millán CG, Lanao JM. Increasing the selectivity of amikacin in rat peritoneal macrophages using carrier erythrocytes. Eur J Pharm Sci. 2009;38(4):320–4.
Millán CG, Castañeda AZ, López FG, Marinero MLS, Lanao JM. Pharmacokinetics and biodistribution of amikacin encapsulated in carrier erythrocytes. J Antimicrob Chemother. 2008;61(2):375–81.
Han X, Shen S, Fan Q, Chen G, Archibong E, Dotti G, Liu Z, Gu Z, Wang C. Red blood cell–derived nanoerythrosome for antigen delivery with enhanced cancer immunotherapy. Science Advances. 2019;5(10):eaaw6870.
Sun X, Han X, Xu L, Gao M, Xu J, Yang R, Liu Z. Surface-Engineering of Red Blood Cells as Artificial Antigen Presenting Cells Promising for Cancer Immunotherapy. Small. 2017;13(40):1701864.
Banz A, Cremel M, Mouvant A, Guerin N, Horand F, Godfrin Y. Tumor Growth Control Using Red Blood Cells as the Antigen Delivery System and Poly(I: C). Journal of Immunotherapy. 2012;35(5).
Sun L, Shen F, Xu J, Han X, Fan C, Liu Z. DNA-Edited Ligand Positioning on Red Blood Cells to Enable Optimized T Cell Activation for Adoptive Immunotherapy. Angew Chem Int Ed. 2020;59(35):14842–53.
McArdel SL, Dugast A-S, Hoover ME, Bollampalli A, Hong E, Castano Z, Leonard SC, Pawar S, Mellen J, Muriuki K, McLaughlin DC, Bayhi N, Carpenter CL, Turka LA, Wickham TJ, Elloul S. Anti-tumor effects of RTX-240: an engineered red blood cell expressing 4–1BB ligand and interleukin-15. Cancer Immunol Immunother. 2021;70(9):2701–19.
Lorentz Kristen M, Kontos S, Diaceri G, Henry H, Hubbell Jeffrey A. Engineered binding to erythrocytes induces immunological tolerance to E. coli asparaginase. Science Advances;1(6):e1500112.
Wang F, Zong R, Chen G. Erythrocyte-enabled immunomodulation for vaccine delivery. J Control Release. 2022;341:314–28.
Aktar N, Chen T, Moudud A, Xu S, Zhou X. Tolerogenic vehicles of antigens in the antigen-specific immunotherapy for autoimmunity. Journal of Drug Delivery Science and Technology. 2021;65: 102772.
Alvarez FJ, Jordán JA, Calleja P, Lotero LA, Olmos G, Díez JC, Tejedor MC. Cross-linking treatment of loaded erythrocytes increases delivery of encapsulated substance to macrophages. Biotechnol Appl Biochem. 1998;27(2):139–43.
Antonelou MH, Kriebardis AG, Papassideri IS. Aging and death signalling in mature red cells: from basic science to transfusion practice. Blood Transfus. 2010;8 Suppl 3(Suppl 3):s39-s47.
Thiagarajan P, Parker CJ, Prchal JT. How Do Red Blood Cells Die? 2021;12.
Lutz HU. Comment Concerning the Role of CD47 and Signal Regulatory Protein Alpha in Regulating the Clearance of Aged Red Blood Cells. Transfusion Medicine and Hemotherapy. 2013;40(2):140–1.
Klei TRL, Dalimot J, Nota B, Veldthuis M, Mul FPJ, Rademakers T, Hoogenboezem M, Nagelkerke SQ, van Ijcken WFJ, Oole E, Svendsen P, Moestrup SK, van Alphen FPJ, Meijer AB, Kuijpers TW, van Zwieten R, van Bruggen R. Hemolysis in the spleen drives erythrocyte turnover. Blood. 2020;136(14):1579–89.
Saleemuddin M, Zimmermann U, Schneeweiß F. Preparation of Human Erythrocyte Ghosts in Isotonic Solution: Haemoglobin Content and Polypeptide Composition. Zeitschrift für Naturforschung C. 1977;32(7–8):627–31.
Tilney LG, Detmers P. Actin in erythrocyte ghosts and its association with spectrin. Evidence for a nonfilamentous form of these two molecules in situ. Journal of Cell Biology. 1975;66(3):508–520.
Ivanov IT, Paarvanova BK, Ivanov V, Smuda K, Bäumler H, Georgieva R. Effects of heat and freeze on isolated erythrocyte submembrane skeletons. Gen Physiol Biophys. 2017;36(2):155–65.
Yuan J, Yin W-y, Wang Y, Chen J, Zhang Z-m, Tang Y-x, Pei S-y, Tan L-x, Hu X-w, Fan X-g, Li N. Cargo-laden erythrocyte ghosts target liver mediated by macrophages. Transfusion and Apheresis Science. 2021;60(1):102930.
Wilson BW, Henderson JD, Ramirez A, O’Malley MA. Standardization of Clinical Cholinesterase Measurements. Int J Toxicol. 2002;21(5):385–8.
Fye HKS, Mrosso P, Bruce L, Thézénas M-L, Davis S, Fischer R, Rwegasira GL, Makani J, Kessler BM. A robust mass spectrometry method for rapid profiling of erythrocyte ghost membrane proteomes. Clin Proteomics. 2018;15(1):14.
Hod EA, Zhang N, Sokol SA, Wojczyk BS, Francis RO, Ansaldi D, Francis KP, Della-Latta P, Whittier S, Sheth S, Hendrickson JE, Zimring JC, Brittenham GM, Spitalnik SL. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115(21):4284–92.
Streuli Ra Fau - Kanofsky JR, Kanofsky Jr Fau - Gunn RB, Gunn Rb Fau - Yachnin S, Yachnin S. Diminished osmotic fragility of human erythrocytes following the membrane insertion of oxygenated sterol compounds. (0006–4971 (Print)).
Dong X, Niu Y, Ding Y, Wang Y, Zhao J, Leng W, Qin L. Formulation and Drug Loading Features of Nano-Erythrocytes. Nanoscale Res Lett. 2017;12(1):202.
Gómez Flores V, Martínez-Martínez A, Roacho Pérez JA, Acosta Bezada J, Aguirre-Tostado FS, García Casillas PE. Biointeraction of Erythrocyte Ghost Membranes with Gold Nanoparticles Fluorescents. Materials. 2021;14(21).
Liu X, Li Y-p, Zhong Z-m, Tan H-q, Lin H-p, Chen S-j, Fu Y-c, Xu W-c, Wei C-j. Incorporation of Viral Glycoprotein VSV-G Improves the Delivery of DNA by Erythrocyte Ghost into Cells Refractory to Conventional Transfection. Applied Biochemistry and Biotechnology. 2017;181(2):748–761.
Okimoto T, Friedmann T, Miyanohara A. VSV-G Envelope Glycoprotein Forms Complexes with Plasmid DNA and MLV Retrovirus-like Particles in Cell-free Conditions and Enhances DNA Transfection. Mol Ther. 2001;4(3):232–8.
Kim S-H, Kim E-J, Hou J-H, Kim J-M, Choi H-G, Shim C-K, Oh Y-K. Opsonized erythrocyte ghosts for liver-targeted delivery of antisense oligodeoxynucleotides. Biomaterials. 2009;30(5):959–67.
Deák R, Mihály J, Szigyártó IC, Beke-Somfai T, Turiák L, Drahos L, Wacha A, Bóta A, Varga Z. Nanoerythrosomes tailoring: Lipid induced protein scaffolding in ghost membrane derived vesicles. Mater Sci Eng, C. 2020;109: 110428.
Deák R, Mihály J, Szigyártó IC, Wacha A, Lelkes G, Bóta A. Physicochemical characterization of artificial nanoerythrosomes derived from erythrocyte ghost membranes. Colloids Surf, B. 2015;135:225–34.
Capossela S, Mathew V, Boos M, Bertolo A, Krupkova O, Stoyanov JV. Novel Fast and Reliable Method for Nano-Erythrosome Production Using Shear Force. Drug Des Devel Ther. 2020;14:4547–60.
Chhabria V, Beeton S. Development of nanosponges from erythrocyte ghosts for removal of streptolysin-O from mammalian blood. Nanomedicine. 2016;11(21):2797–807.
AlQahtani SA, Harisa GI, Alomrani AH, Alanazi FK, Badran MM. Improved pharmacokinetic and biodistribution of 5-fluorouracil loaded biomimetic nanoerythrocytes decorated nanocarriers for liver cancer treatment. Colloids Surf, B. 2021;197: 111380.
Berikkhanova K, Omarbaev R, Gulyayev A, Shulgau Z, Ibrasheva D, Adilgozhina G, Sergazy S, Zhumadilov Z, Askarova S. Red blood cell ghosts as promising drug carriers to target wound infections. Med Eng Phys. 2016;38(9):877–84.
Hod EA, Brittenham Gm Fau - Billote GB, Billote Gb Fau - Francis RO, Francis Ro Fau - Ginzburg YZ, Ginzburg Yz Fau - Hendrickson JE, Hendrickson Je Fau - Jhang J, Jhang J Fau - Schwartz J, Schwartz J Fau - Sharma S, Sharma S Fau - Sheth S, Sheth S Fau - Sireci AN, Sireci An Fau - Stephens HL, Stephens Hl Fau - Stotler BA, Stotler Ba Fau - Wojczyk BS, Wojczyk Bs Fau - Zimring JC, Zimring Jc Fau - Spitalnik SL, Spitalnik SL. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. (1528–0020 (Electronic)).
Mambrini G, Mandolini M, Rossi L, Pierigè F, Capogrossi G, Salvati P, Serafini S, Benatti L, Magnani M. Ex vivo encapsulation of dexamethasone sodium phosphate into human autologous erythrocytes using fully automated biomedical equipment. Int J Pharm. 2017;517(1):175–84.
Boogaard C, Dixon GJECR. Red cell ghost-mediated microinjection of RNA into HeLa cells: I. A comparison of two techniques for the entrapment and microinjection of tRNA and mRNA. 1983;143(1):175–190.
Antonelli A, Sfara C, Manuali E, Bruce IJ, Magnani M. Encapsulation of superparamagnetic nanoparticles into red blood cells as new carriers of MRI contrast agents. Nanomedicine. 2011;6(2):211–23.
Rahman MH, Wong CHN, Lee MM, Chan MK, Ho Y-P. Efficient encapsulation of functional proteins into erythrocytes by controlled shear-mediated membrane deformation. Lab Chip. 2021;21(11):2121–8.
Green R, Lamon J, Curran D. Clinical trial of desferrioxamine entrapped in red cell ghosts. The Lancet. 1980;316(8190):327–30.
Malhotra S, Dumoga S, Singh N. Red blood cells membrane-derived nanoparticles: Applications and key challenges in their clinical translation. WIREs Nanomedicine and Nanobiotechnology. 2022;n/a(n/a):e1776.
Pan Y, Wang F, Liu Y, Jiang J, Yang Y-G, Wang H. Studying the mechanism of CD47–SIRPα interactions on red blood cells by single molecule force spectroscopy. Nanoscale. 2014;6(17):9951–4.
Hu Che-Ming J, Zhang L, Aryal S, Cheung C, Fang Ronnie H, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci. 2011;108(27):10980–5.
Gao W, Hu C-MJ, Fang RH, Luk BT, Su J, Zhang L. Surface Functionalization of Gold Nanoparticles with Red Blood Cell Membranes. Advanced Materials. 2013;25(26):3549–3553.
Li S, Zhang L. Erythrocyte membrane nano-capsules: biomimetic delivery and controlled release of photothermal–photochemical coupling agents for cancer cell therapy. Dalton Trans. 2020;49(8):2645–51.
Ran L, Lu B, Qiu H, Zhou G, Jiang J, Hu E, Dai F, Lan G. Erythrocyte membrane-camouflaged nanoworms with on-demand antibiotic release for eradicating biofilms using near-infrared irradiation. Bioactive Materials. 2021;6(9):2956–68.
Ren X, Zheng R, Fang X, Wang X, Zhang X, Yang W, Sha X. Red blood cell membrane camouflaged magnetic nanoclusters for imaging-guided photothermal therapy. Biomaterials. 2016;92:13–24.
Rao L, Cai B, Bu L-L, Liao Q-Q, Guo S-S, Zhao X-Z, Dong W-F, Liu W. Microfluidic Electroporation-Facilitated Synthesis of Erythrocyte Membrane-Coated Magnetic Nanoparticles for Enhanced Imaging-Guided Cancer Therapy. ACS Nano. 2017;11(4):3496–505.
Lin K, Cao Y, Zheng D, Li Q, Liu H, Yu P, Li J, Xue Y, Wu M. Facile phase transfer of hydrophobic Fe3O4@Cu2−xS nanoparticles by red blood cell membrane for MRI and phototherapy in the second near-infrared window. Journal of Materials Chemistry B. 2020;8(6):1202–11.
Jakobsson U, Mäkilä E, Rahikkala A, Imlimthan S, Lampuoti J, Ranjan S, Heino J, Jalkanen P, Köster U, Mizohata K, Santos HA, Salonen J, Airaksinen AJ, Sarparanta M, Helariutta K. Preparation and in vivo evaluation of red blood cell membrane coated porous silicon nanoparticles implanted with 155Tb. Nucl Med Biol. 2020;84–85:102–10.
Peng H, Xu Z, Wang Y, Feng N, Yang W, Tang J. Biomimetic Mesoporous Silica Nanoparticles for Enhanced Blood Circulation and Cancer Therapy. ACS Appl Bio Mater. 2020;3(11):7849–57.
Nazemidashtarjandi S, Farnoud AM. Membrane outer leaflet is the primary regulator of membrane damage induced by silica nanoparticles in vesicles and erythrocytes. Environ Sci Nano. 2019;6(4):1219–32.
Li M, Fang H, Liu Q, Gai Y, Yuan L, Wang S, Li H, Hou Y, Gao M, Lan X. Red blood cell membrane-coated upconversion nanoparticles for pretargeted multimodality imaging of triple-negative breast cancer. Biomaterials Science. 2020;8(7):1802–14.
Rao L, Meng Q-F, Bu L-L, Cai B, Huang Q, Sun Z-J, Zhang W-F, Li A, Guo S-S, Liu W, Wang T-H, Zhao X-Z. Erythrocyte Membrane-Coated Upconversion Nanoparticles with Minimal Protein Adsorption for Enhanced Tumor Imaging. ACS Appl Mater Interfaces. 2017;9(3):2159–68.
Ding H, Lv Y, Ni D, Wang J, Tian Z, Wei W, Ma G. Erythrocyte membrane-coated NIR-triggered biomimetic nanovectors with programmed delivery for photodynamic therapy of cancer. Nanoscale. 2015;7(21):9806–15.
Wang Z, Cheng L, Sun Y, Wei X, Cai B, Liao L, Zhang Y, Zhao X-Z. Enhanced Isolation of Fetal Nucleated Red Blood Cells by Enythrocyte-Leukocyte Hybrid Membrane-Coated Magnetic Nanoparticles for Noninvasive Pregnant Diagnostics. Anal Chem. 2021;93(2):1033–42.
Zou S, Wang B, Wang C, Wang Q, Zhang L. Cell membrane-coated nanoparticles: research advances. Nanomedicine. 2020;15(6):625–41.
Zhuang J, Duan Y, Zhang Q, Gao W, Li S, Fang RH, Zhang L. Multimodal enzyme delivery and therapy enabled by cell membrane-coated metal-organic framework nanoparticles. Nano Lett. 2020;20(5):4051–8.
Liu X, Jansman MMT, Hosta-Rigau L. Haemoglobin-loaded metal organic framework-based nanoparticles camouflaged with a red blood cell membrane as potential oxygen delivery systems. Biomaterials Science. 2020;8(21):5859–73.
Gao M, Liang C, Song X, Chen Q, Jin Q, Wang C, Liu Z. Erythrocyte-membrane-enveloped perfluorocarbon as nanoscale artificial red blood cells to relieve tumor hypoxia and enhance cancer radiotherapy. Adv Mater. 2017;29(35):1701429.
Ren H, Liu J, Li Y, Wang H, Ge S, Yuan A, Hu Y, Wu J. Oxygen self-enriched nanoparticles functionalized with erythrocyte membranes for long circulation and enhanced phototherapy. Acta Biomater. 2017;59:269–82.
Zhang D, Zhou C, Liu F, Ding T, Wang Z. Red blood cells membrane vehicle co-delivering DOX and IR780 for effective prostate cancer therapy. J Mater Res. 2020;35(22):3116–23.
Wan G, Chen B, Li L, Wang D, Shi S, Zhang T, Wang Y, Zhang L, Wang Y. Nanoscaled red blood cells facilitate breast cancer treatment by combining photothermal/photodynamic therapy and chemotherapy. Biomaterials. 2018;155:25–40.
Qian H, Qian K, Cai J, Yang Y, Zhu L, Liu B. Therapy for gastric cancer with peritoneal metastasis using injectable albumin hydrogel hybridized with paclitaxel-loaded red blood cell membrane nanoparticles. ACS Biomater Sci Eng. 2019;5(2):1100–12.
Chai Z, Hu X, Wei X, Zhan C, Lu L, Jiang K, Su B, Ruan H, Ran D, Fang RH, Zhang L, Lu W. A facile approach to functionalizing cell membrane-coated nanoparticles with neurotoxin-derived peptide for brain-targeted drug delivery. J Control Release. 2017;264:102–11.
Guo Y, Wang D, Song Q, Wu T, Zhuang X, Bao Y, Kong M, Qi Y, Tan S, Zhang Z. Erythrocyte membrane-enveloped polymeric nanoparticles as nanovaccine for induction of antitumor immunity against melanoma. ACS Nano. 2015;9(7):6918–33.
Liu W, Ruan M, Wang Y, Song R, Ji X, Xu J, Dai J, Xue W. Light-Triggered biomimetic nanoerythrocyte for tumor-targeted lung metastatic combination therapy of malignant melanoma. Small. 2018;14(38):1801754.
Tan S, Wu T, Zhang D, Zhang Z. Cell or cell membrane-based drug delivery systems. Theranostics. 2015;5(8):863–81.
Sun R, Ge Y, Liu H, He P, Song W, Zhang X. Erythrocyte Membrane-Encapsulated Glucose Oxidase and Manganese/Ferrite Nanocomposite as a Biomimetic “All in One” Nanoplatform for Cancer Therapy. ACS Appl Bio Mater. 2021;4(1):701–10.
Aryal S, Hu C-MJ, Fang RH, Dehaini D, Carpenter C, Zhang D-E, Zhang L. Erythrocyte membrane-cloaked polymeric nanoparticles for controlled drug loading and release. Nanomedicine. 2013;8(8):1271–1280.
Zhang Z, Qian H, Yang M, Li R, Hu J, Li L, Yu L, Liu B, Qian X. Gambogic acid-loaded biomimetic nanoparticles in colorectal cancer treatment. Int J Nanomedicine. 2017;12:1593–605.
Sathiyaseelan A, Saravanakumar K, Manivasagan P, Jeong MS, Jang E-S, Wang M-H. Folic acid conjugated chitosan encapsulated palladium nanoclusters for NIR triggered photothermal breast cancer treatment. Carbohyd Polym. 2022;280: 119021.
Huang X, Wu B, Li J, Shang Y, Chen W, Nie X, Gui RA-O. Anti-tumour effects of red blood cell membrane-camouflaged black phosphorous quantum dots combined with chemotherapy and anti-inflammatory therapy. (2169–141X (Electronic)).
Liang X, Li H, Zhang A, Tian X, Guo H, Zhang H, Yang J, Zeng Y. Red blood cell biomimetic nanoparticle with anti-inflammatory, anti-oxidative and hypolipidemia effect ameliorated atherosclerosis therapy. Nanomedicine: Nanotechnology, Biology and Medicine. 2022;41:102519.
Xue X, Liu H, Wang S, Hu Y, Huang B, Li M, Gao J, Wang X, Su J. Neutrophil-erythrocyte hybrid membrane-coated hollow copper sulfide nanoparticles for targeted and photothermal/ anti-inflammatory therapy of osteoarthritis. Compos B Eng. 2022;237: 109855.
Liang H, Huang K, Su T, Li Z, Hu S, Dinh P-U, Wrona EA, Shao C, Qiao L, Vandergriff AC, Hensley MT, Cores J, Allen T, Zhang H, Zeng Q, Xing J, Freytes DO, Shen D, Yu Z, Cheng K. Mesenchymal stem cell/red blood cell-inspired nanoparticle therapy in mice with carbon tetrachloride-induced acute liver failure. ACS Nano. 2018;12(7):6536–44.
Hayashi K, Yamada S, Hayashi H, Sakamoto W, Yogo T. Red blood cell-like particles with the ability to avoid lung and spleen accumulation for the treatment of liver fibrosis. Biomaterials. 2018;156:45–55.
Wu X, Zhang X, Feng W, Feng H, Ding Z, Zhao Q, Li X, Tang N, Zhang P, Li J, Wang J. A targeted erythrocyte membrane-encapsulated drug-delivery system with anti-osteosarcoma and anti-osteolytic effects. ACS Appl Mater Interfaces. 2021;13(24):27920–33.
Xuan M, Shao J, Zhao J, Li Q, Dai L, Li J. Magnetic mesoporous silica nanoparticles cloaked by red blood cell membranes: applications in cancer therapy. Angew Chem Int Ed. 2018;57(21):6049–53.
Biagiotti S, Paoletti MF, Fraternale A, Rossi L, Magnani M. Drug delivery by red blood cells. IUBMB Life. 2011;63(8):621–31.
Hui L, Qin S, Yang L. Upper critical solution temperature polymer, photothermal agent, and erythrocyte membrane coating: an unexplored recipe for making drug carriers with spatiotemporally controlled cargo release. ACS Biomater Sci Eng. 2016;2(12):2127–32.
Su J, Sun H, Meng Q, Zhang P, Yin Q, Li Y. Enhanced blood suspensibility and laser-activated tumor-specific drug release of theranostic mesoporous silica nanoparticles by functionalizing with erythrocyte membranes. Theranostics. 2017;7(3):523–37.
Hsieh C-C, Kang S-T, Lin Y-H, Ho Y-J, Wang C-H, Yeh C-K, Chang C-W. Biomimetic acoustically-responsive vesicles for theranostic applications. Theranostics. 2015;5(11):1264–74.
Zhang H, Liang Y, Zhao H, Qi R, Chen Z, Yuan H, Liang H, Wang L. Dual-mode antibacterial conjugated polymer nanoparticles for photothermal and photodynamic therapy. Macromol Biosci. 2020;20(2):1900301.
Sun D, Chen J, Wang Y, Ji H, Peng R, Jin L, Wu W. Advances in refunctionalization of erythrocyte-based nanomedicine for enhancing cancer-targeted drug delivery. Theranostics. 2019;9(23):6885–900.
Chen W, Zeng K, Liu H, Ouyang J, Wang L, Liu Y, Wang H, Deng L, Liu Y-N. Cell membrane camouflaged hollow prussian blue nanoparticles for synergistic photothermal-/chemotherapy of cancer. Adv Func Mater. 2017;27(11):1605795.
Pei Q, Hu X, Zheng X, Liu S, Li Y, Jing X, Xie Z. Light-activatable red blood cell membrane-camouflaged dimeric prodrug nanoparticles for synergistic photodynamic/chemotherapy. ACS Nano. 2018;12(2):1630–41.
Li Q, Lin B, Li Y, Lu N. Erythrocyte-camouflaged mesoporous titanium dioxide nanoplatform for an ultrasound-mediated sequential therapies of breast cancer. Int J Nanomedicine. 2021;16:3875–87.
Zhang H, Chen J, Zhu X, Ren Y, Cao F, Zhu L, Hou L, Zhang H, Zhang Z. Ultrasound induced phase-transition and invisible nanobomb for imaging-guided tumor sonodynamic therapy. Journal of Materials Chemistry B. 2018;6(38):6108–21.
Feng X, Ma L, Lei J, Ouyang Q, Zeng Y, Luo Y, Zhang X, Song Y, Li G, Tan L, Liu X, Yang C. Piezo-augmented sonosensitizer with strong ultrasound-propelling ability for efficient treatment of osteomyelitis. ACS Nano. 2022;16(2):2546–57.
Yan J, Yu J, Wang C, Gu Z. Red blood cells for drug delivery. Small Methods. 2017;1(12):1700270.
Ye S, Wang F, Fan Z, Zhu Q, Tian H, Zhang Y, Jiang B, Hou Z, Li Y, Su G. Light/pH-triggered biomimetic red blood cell membranes camouflaged small molecular drug assemblies for imaging-guided combinational chemo-photothermal therapy. ACS Appl Mater Interfaces. 2019;11(17):15262–75.
Lai P-Y, Huang R-Y, Lin S-Y, Lin Y-H, Chang C-W. Biomimetic stem cell membrane-camouflaged iron oxide nanoparticles for theranostic applications. RSC Adv. 2015;5(119):98222–30.
Sun X, Wang C, Gao M, Hu A, Liu Z. Remotely Controlled Red Blood Cell Carriers for Cancer Targeting and Near-Infrared Light-Triggered Drug Release in Combined Photothermal-Chemotherapy. Adv Func Mater. 2015;25(16):2386–94.
Fang RH, Jiang Y, Fang JC, Zhang L. Cell membrane-derived nanomaterials for biomedical applications. Biomaterials. 2017;128:69–83.
Bidkar AP, Sanpui P, Ghosh SS. Transferrin-Conjugated Red Blood Cell Membrane-Coated Poly(lactic-co-glycolic acid) Nanoparticles for the Delivery of Doxorubicin and Methylene Blue. ACS Applied Nano Materials. 2020;3(4):3807–19.
Chen H-Y, Deng J, Wang Y, Wu C-Q, Li X, Dai H-W. Hybrid cell membrane-coated nanoparticles: A multifunctional biomimetic platform for cancer diagnosis and therapy. Acta Biomater. 2020;112:1–13.
Chen Z, Wang W, Li Y, Wei C, Zhong P, He D, Liu H, Wang P, Huang Z, Zhu W, Zhou Y, Qin L. Folic acid-modified erythrocyte membrane loading dual drug for targeted and chemo-photothermal synergistic cancer therapy. Mol Pharm. 2021;18(1):386–402.
Falsafi M, Zahiri M, Saljooghi AS, Abnous K, Taghdisi SM, Sazgarnia A, Ramezani M, Alibolandi M. Aptamer targeted red blood cell membrane-coated porphyrinic copper-based MOF for guided photochemotherapy against metastatic breast cancer. Microporous Mesoporous Mater. 2021;325: 111337.
Zhao Y, Wang J, Cai X, Ding P, Lv H, Pei R. Metal-organic frameworks with enhanced photodynamic therapy: Synthesis, erythrocyte membrane camouflage, and aptamer-targeted aggregation. ACS Appl Mater Interfaces. 2020;12(21):23697–706.
Zhang C, Zhang P-Q, Guo S, Chen G, Zhao Z, Wang G-X, Zhu B. Application of Biomimetic Cell-Derived Nanoparticles with Mannose Modification as a Novel Vaccine Delivery Platform against Teleost Fish Viral Disease. ACS Biomater Sci Eng. 2020;6(12):6770–7.
Zhu DM, Xie W Fau - Xiao Y-S, Xiao Ys Fau - Suo M, Suo M Fau - Zan M-H, Zan Mh Fau - Liao Q-Q, Liao Qq Fau - Hu X-J, Hu Xj Fau - Chen L-B, Chen Lb Fau - Chen B, Chen B Fau - Wu W-T, Wu Wt Fau - Ji L-W, Ji Lw Fau - Huang H-M, Huang Hm Fau - Guo S-S, Guo Ss Fau - Zhao X-Z, Zhao Xz Fau - Liu Q-Y, Liu Qy Fau - Liu W, Liu W. Erythrocyte membrane-coated gold nanocages for targeted photothermal and chemical cancer therapy. (1361–6528 (Electronic)).
Mao J, Bian Y, Zhang Q, Kong L, Shi X, Hu J, Yang M, Li L, Qian H, Liu B, Qian X. Antitumor activity of iRGD-modified red blood cell membrane nanoparticles loaded with Juglone and Oxaliplatin against colorectal cancer. J Biomater Appl. 2021;36(7):1301–16.
Fu Q, Lv P, Chen Z, Ni D, Zhang L, Yue H, Yue Z, Wei W, Ma G. Programmed co-delivery of paclitaxel and doxorubicin boosted by camouflaging with erythrocyte membrane. Nanoscale. 2015;7(9):4020–30.
Gao W, Zhang LJAJ. Engineering re„ bloo„ cell membrane coated nanoparticles for broad biomedical applications. 2015;61:738–746.
Chai Z, Ran D, Lu L, Zhan C, Ruan H, Hu X, Xie C, Jiang K, Li J, Zhou J, Wang J, Zhang Y, Fang RH, Zhang L, Lu W. Ligand-Modified Cell Membrane Enables the Targeted Delivery of Drug Nanocrystals to Glioma. ACS Nano. 2019;13(5):5591–601.
Fu S, Liang M, Wang Y, Cui L, Gao C, Chu X, Liu Q, Feng Y, Gong W, Yang M, Li Z, Yang C, Xie X, Yang Y, Gao C. Dual-Modified Novel Biomimetic Nanocarriers Improve Targeting and Therapeutic Efficacy in Glioma. ACS Appl Mater Interfaces. 2019;11(2):1841–54.
Li H, Peng Q, Yang L, Lin Y, Chen S, Qin Y, Li S, Yu X, Zhang L. High-Performance Dual Combination Therapy for Cancer Treatment with Hybrid Membrane-Camouflaged Mesoporous Silica Gold Nanorods. ACS Appl Mater Interfaces. 2020;12(52):57732–45.
Jiao X, Yu X, Gong C, Zhu H, Zhang B, Wang R, Yuan YJCpb. Erythrocyte-cancer hybrid membrane-camouflaged mesoporous silica nanoparticles loaded with Gboxin for glioma-targeting therapy. 2022.
Shan X, Zhang C, Mai C, Hu X, Cheng N, Chen W, Peng D, Wang L, Ji Z, Xie Y. The Biogenesis, Biological Functions, and Applications of Macrophage-Derived Exosomes. 2021;8.
Dehaini D, Wei X, Fang RH, Masson S, Angsantikul P, Luk BT, Zhang Y, Ying M, Jiang Y, Kroll AV, Gao W, Zhang L. Erythrocyte-Platelet Hybrid Membrane Coating for Enhanced Nanoparticle Functionalization. Adv Mater. 2017;29(16):1606209.
Esteban-Fernández de Ávila B, Angsantikul P, Ramírez-Herrera Doris E, Soto F, Teymourian H, Dehaini D, Chen Y, Zhang L, Wang J. Hybrid biomembrane–functionalized nanorobots for concurrent removal of pathogenic bacteria and toxins. Science Robotics. 2018;3(18):eaat0485.
You P, Mayier A, Zhou H, Yang A, Fan J, Ma S, Liu B, Jiang Y. Targeting and promoting atherosclerosis regression using hybrid membrane coated nanomaterials via alleviated inflammation and enhanced autophagy. Appl Mater Today. 2022;26: 101386.
Xiong J, Wu M, Chen J, Liu Y, Chen Y, Fan G, Liu Y, Cheng J, Wang Z, Wang S, Liu Y, Zhang W. Cancer-Erythrocyte Hybrid Membrane-Camouflaged Magnetic Nanoparticles with Enhanced Photothermal-Immunotherapy for Ovarian Cancer. ACS Nano. 2021;15(12):19756–70.
Cheng H, Fan J-H, Zhao L-P, Fan G-L, Zheng R-R, Qiu X-Z, Yu X-Y, Li S-Y, Zhang X-Z. Chimeric peptide engineered exosomes for dual-stage light guided plasma membrane and nucleus targeted photodynamic therapy. Biomaterials. 2019;211:14–24.
Wang Y, Zhang K, Qin X, Li T, Qiu J, Yin T, Huang J, McGinty S, Pontrelli G, Ren J, Wang Q, Wu W, Wang G. Biomimetic Nanotherapies: Red Blood Cell Based Core-Shell Structured Nanocomplexes for Atherosclerosis Management. Advanced Science. 2019;6(12):1900172.
Shao J, Abdelghani M, Shen G, Cao S, Williams DS, van Hest JCM. Erythrocyte membrane modified janus polymeric motors for thrombus therapy. ACS Nano. 2018;12(5):4877–85.
Rossi L, Fraternale A, Bianchi M, Magnani M. Red Blood Cell Membrane Processing for Biomedical Applications. 2019;10.
Li M, Xu Z, Zhang L, Cui M, Zhu M, Guo Y, Sun R, Han J, Song E, He Y, Su Y. Targeted noninvasive treatment of choroidal neovascularization by hybrid cell-membrane-cloaked biomimetic nanoparticles. ACS Nano. 2021;15(6):9808–19.
Wang Y, Luan Z, Zhao C, Bai C, Yang K. Target delivery selective CSF-1R inhibitor to tumor-associated macrophages via erythrocyte-cancer cell hybrid membrane camouflaged pH-responsive copolymer micelle for cancer immunotherapy. Eur J Pharm Sci. 2020;142: 105136.
Kalluri R, LeBleu Valerie S. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977.
Wiklander Oscar PB, Brennan Meadhbh Á, Lötvall J, Breakefield Xandra O, Samir ELA. Advances in therapeutic applications of extracellular vesicles. Science Translational Medicine. 2019;11(492):eaav8521.
Costa Verdera H, Gitz-Francois JJ, Schiffelers RM, Vader P. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J Control Release. 2017;266:100–8.
Chiangjong W, Netsirisawan P, Hongeng S, Chutipongtanate S. Red Blood Cell Extracellular Vesicle-Based Drug Delivery: Challenges and Opportunities. 2021;8.
Keles E, Song Y, Du D, Dong W-J, Lin Y. Recent progress in nanomaterials for gene delivery applications. Biomaterials Science. 2016;4(9):1291–309.
Farris E, Heck K, Lampe AT, Brown DM, Ramer-Tait AE, Pannier AK. Oral non-viral gene delivery for applications in DNA vaccination and gene therapy. Current Opinion in Biomedical Engineering. 2018;7:51–7.
Rey-Rico A, Cucchiarini M. Controlled release strategies for rAAV-mediated gene delivery. Acta Biomater. 2016;29:1–10.
Zhao Z, Anselmo AC, Mitragotri S. Viral vector-based gene therapies in the clinic. Bioengineering & Translational Medicine. 2022;7(1): e10258.
Wan C, Allen TM, Cullis PR. Lipid nanoparticle delivery systems for siRNA-based therapeutics. Drug Deliv Transl Res. 2014;4(1):74–83.
Usman WM, Pham TC, Kwok YY, Vu LT, Ma V, Peng B, Chan YS, Wei L, Chin SM, Azad A, He AB-L, Leung AYH, Yang M, Shyh-Chang N, Cho WC, Shi J, Le MTN. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nature Communications. 2018;9(1):2359.
Zhang G, Huang X, Xiu H, Sun Y, Chen J, Cheng G, Song Z, Peng Y, Shen Y, Wang J, Cai Z. Extracellular vesicles: Natural liver-accumulating drug delivery vehicles for the treatment of liver diseases. Journal of Extracellular Vesicles. 2020;10(2): e12030.
Chang M, Hsiao J-K, Yao M, Chien L-Y, Hsu S-C, Ko B-S, Chen S-T, Liu H-M, Chen Y-C, Yang C-SJN. Homologous RBC-derived vesicles as ultrasmall carriers of iron oxide for magnetic resonance imaging of stem cells. 2010;21(23):235103.
Borgheti-Cardoso LN, Kooijmans SAA, Chamorro LG, Biosca A, Lantero E, Ramírez M, Avalos-Padilla Y, Crespo I, Fernández I, Fernandez-Becerra C, del Portillo HA, Fernàndez-Busquets X. Extracellular vesicles derived from Plasmodium-infected and non-infected red blood cells as targeted drug delivery vehicles. Int J Pharm. 2020;587: 119627.
Kuo WP, Tigges JC, Toxavidis V, Ghiran I. Red Blood Cells: A Source of Extracellular Vesicles. In: Kuo WP, Jia S, editors. Extracellular Vesicles: Methods and Protocols. New York, NY: Springer New York; 2017. p. 15–22.
Huber HJ, Holvoet P. Exosomes: emerging roles in communication between blood cells and vascular tissues during atherosclerosis. Curr Opin Lipidol. 2015;26(5):412–9.
Davidson SM, Andreadou I, Barile L, Birnbaum Y, Cabrera-Fuentes HA, Cohen MV, Downey JM, Girao H, Pagliaro P, Penna C, Pernow J, Preissner KT, Ferdinandy P, on behalf of the EUCCA. Circulating blood cells and extracellular vesicles in acute cardioprotection. Cardiovascular Research. 2019;115(7):1156–1166.
Thangaraju K, Neerukonda SN, Katneni U, Buehler PW. Extracellular Vesicles from Red Blood Cells and Their Evolving Roles in Health, Coagulopathy and Therapy. International Journal of Molecular Sciences. 2021;22(1).
Gao Y, Jin H, Tan H, Wang Y, Wu J, Wang Y, Zhang J, Yang Y, Tian W, Hou R. The role of extracellular vesicles from stored RBC units in B lymphocyte survival and plasma cell differentiation. J Leukoc Biol. 2020;108(6):1765–76.
Sadallah S, Eken C, Schifferli JA. Erythrocyte-derived ectosomes have immunosuppressive properties. J Leukoc Biol. 2008;84(5):1316–25.
Straat M, Böing AN, Tuip-De Boer A, Nieuwland R, Juffermans NP. Extracellular Vesicles from Red Blood Cell Products Induce a Strong Pro-Inflammatory Host Response Dependent on Both Numbers and Storage Duration. Transfusion Medicine and Hemotherapy. 2016;43(4):302–5.
Gangadaran P, Hong CM, Oh JM, Rajendran RL, Kalimuthu S, Son SH, Gopal A, Zhu L, Baek SH, Jeong SY, Lee S-W, Lee J, Ahn B-C. In vivo Non-invasive Imaging of Radio-Labeled Exosome-Mimetics Derived From Red Blood Cells in Mice. 2018;9.
Yoshida T, Prudent M, D’Alessandro A. Red blood cell storage lesion: causes and potential clinical consequences. Blood Transfus. 2019;17(1):27–52.
Crowe JH, Crowe LM, Carpenter JF, Aurell WC. Stabilization of dry phospholipid bilayers and proteins by sugars. Biochem J. 1987;242(1):1–10.
Sun WQ, Leopold AC, Crowe LM, Crowe JH. Stability of dry liposomes in sugar glasses. Biophys J. 1996;70(4):1769–76.
Gupta A, Das R, Makabenta JM, Gupta A, Zhang X, Jeon T, Huang R, Liu Y, Gopalakrishnan S, Milán R-C, Rotello VM. Erythrocyte-mediated delivery of bioorthogonal nanozymes for selective targeting of bacterial infections. Mater Horiz. 2021;8(12):3424–31.
Patel J, Chowdhury EA, Noorani B, Bickel U, Huang J. Isoflurane increases cell membrane fluidity significantly at clinical concentrations. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2020;1862(2):183140.
Wang C, Ye Y, Sun W, Yu J, Wang J, Lawrence DS, Buse JB, Gu Z. Red Blood Cells for Glucose-Responsive Insulin Delivery. Adv Mater. 2017;29(18):1606617.
Acknowledgements
ZZ acknowledge support from College of Pharmacy at University of Illinois Chicago.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, E., Phan, P., Algarni, H.A. et al. Red Blood Cell Inspired Strategies for Drug Delivery: Emerging Concepts and New Advances. Pharm Res 39, 2673–2698 (2022). https://doi.org/10.1007/s11095-022-03328-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03328-5