Abstract
Purpose
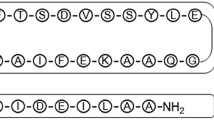
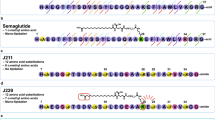
Semaglutide is the only oral GLP-1 RA in the market, but oral bioavailability is generally limited in range of 0.4–1%. In this study, a new GLP-1RA named SHR-2042 was developed to gain higher oral bioavailability than semaglutide.
Method
Self-association of SHR-2042, semaglutide and liraglutide were assessed using SEC-MALS. The intestinal perfusion test in SD rats was used to select permeation enhancers (PEs) including SNAC, C10 and LCC. ITC, CD and DLS were used to explore the interaction between SHR-2042 and SNAC. Gastric administrated test in SD rats was used to screen SHR-2042 granules with different SHR-2042/SNAC ratios. The oral bioavailability of SHR-2042 was studied in rats and monkeys.
Result
The designed GLP-1RA, SHR-2042, gives a better solubility and lipophilicity than semaglutide. While it forms a similar oligomer with that of semaglutide. During the selection of PEs, SNAC shows better exposure than the other competing PEs including C10 and LCC. SHR-2042 and SNAC bind quickly and exhibit hydrophobic interaction. SNAC could promote monomerization of SHR-2042 and form micelles to trap the monomerized SHR-2042. The oral bioavailability of SHR-2042 paired with SNAC is 0.041% (1:0, w/w), 0.083% (1:10, w/w), 0.32% (1:30, w/w) and 2.83% (1:60, w/w) in rats. And the oral bioavailability of SHR-2042 matched with SNAC is 3.39% (1:30, w/w) in monkeys, which is over 10 times higher than that of semaglutide.
Conclusion
We believe that the design and development of oral SHR-2042 will provide a new way to design more and more GLP-1RAs with high oral bioavailability in the future.













Similar content being viewed by others
Abbreviations
- GLP-1:
-
Glucagon-like peptide-1
- GLP-1RAs:
-
Glucagon-like peptide-1 receptor agonists
- T2DM:
-
Type 2 diabetes mellitus
- ADO:
-
Dioxaoctanoic acid
- SEC-MALS:
-
Size-exclusion chromatography-multiangle laser scattering
- ITC:
-
Isothermal titration calorimetry
- CD:
-
Circular dichroism
- DLS:
-
Dynamic Light Scattering
- PEs:
-
Permeation enhancers
- SNAC:
-
Sodium N-(8-[2-hydroxybenzoyl] amino) caprylate
- C10:
-
Sodium decanoate
- LCC:
-
L-lauroylcarnitine chloride
- SD rats:
-
Sprague-Dawley rats
- RP-HPLC:
-
Reversed phase -high performance liquid chromatography
- QC:
-
Quality control
- NCA:
-
Non-compartmental model analysis
- Tmax :
-
Peak time
- Cmax :
-
Maximum concentration
- AUC:
-
Area under the concentration–time curve
- t1/2 :
-
Elimination half-life
- Vd :
-
Apparent volume of distribution
- Cl:
-
Plasma clearance
- F:
-
Bioavailability
- SD:
-
Standard deviation
- CMC:
-
Critical micelle concentration
- PBS:
-
Phosphate buffer solution
References
Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136(8):3585–96.
Vilsbøll T, Agersø H, Krarup T, Holst JJ. Similar elimination rates of glucagon-like peptide-1 in obese type 2 diabetic patients and healthy subjects. J Clin Endocrinol Metab. 2003;88(1):220–4.
Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773–95.
Koliaki C, Doupis J. Incretin-based therapy: a powerful and promising weapon in the treatment of type 2 diabetes mellitus. Diabetes Ther. 2011;2(2):101–21.
Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2016;18(3):203–16.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–705.
Htike ZZ, Zaccardi F, Papamargaritis D, Webb DR, Khunti K, Davies MJ. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: A systematic review and mixed-treatment comparison analysis. Diabetes Obes Metab. 2017;19(4):524–36.
Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diab Care. 2018;41(12):2669–701.
Goldberg M, Gomez-Orellana I. Challenges for the oral delivery of macromolecules. Nat Rev Drug Discov. 2003;2(4):289–95.
Hamman JH, Enslin GM, Kotzé AF. Oral delivery of peptide drugs: barriers and developments. BioDrugs. 2005;19(3):165–77.
Bruno BJ, Miller GD, Lim CS. Basics and recent advances in peptide and protein drug delivery. Ther Deliv. 2013;4(11):1443–67.
Buckley ST, Bækdal TA, Vegge A, Maarbjerg SJ, Pyke C, Ahnfelt-Rønne J, Madsen KG, Schéele SG, Alanentalo T, Kirk RK, Pedersen BL, Skyggebjerg RB, Benie AJ, Strauss HM, Wahlund PO, Bjerregaard S, Farkas E, Fekete C, Søndergaard FL, Borregaard J, Hartoft-Nielsen ML, Knudsen LB. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci Transl Med. 2018;10(467):eaar7047.
Djb A, Tah B, Dpf B, Sm C, Rjm DJADDR. Systemic delivery of peptides by the oral route: Formulation and medicinal chemistry approaches - ScienceDirect. 2020;157:2–36.
Russell-Jones D. Molecular, pharmacological and clinical aspects of liraglutide, a once-daily human GLP-1 analogue. Mol Cell Endocrinol. 2009;297(1–2):137–40.
Lau J, Bloch P, Schäffer L, Pettersson I, Spetzler J, Kofoed J, Madsen K, Knudsen LB, McGuire J, Steensgaard DB, Strauss HM, Gram DX, Knudsen SM, Nielsen FS, Thygesen P, Reedtz-Runge S, Kruse T. Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) Analogue Semaglutide. J Med Chem. 2015;58(18):7370–80.
Madsen K, Knudsen LB, Agersoe H, Nielsen PF, Thøgersen H, Wilken M, Johansen NL. Structure-activity and protraction relationship of long-acting glucagon-like peptide-1 derivatives: importance of fatty acid length, polarity, and bulkiness. J Med Chem. 2007;50(24):6126–32.
Steensgaard DB, Thomsen JK, Olsen HB, Knudsen LBJD. The Molecular Basis for the Delayed Absorption of the Once-Daily Human GLP-1 Analoge. Liraglutide. 2008;57:A164.
Liu X, Testa B, Fahr A. Lipophilicity and its relationship with passive drug permeation. Pharm Res. 2011;28(5):962–77.
Ditzinger F, Price DJ, Ilie AR, Köhl NJ, Jankovic S, Tsakiridou G, Aleandri S, Kalantzi L, Holm R, Nair A, Saal C, Griffin B, Kuentz M. Lipophilicity and hydrophobicity considerations in bio-enabling oral formulations approaches - a PEARRL review. J Pharm Pharmacol. 2019;71(4):464–82.
Göke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J, Göke B. Exendin-4 is a high potency agonist and truncated exendin-(9-39)-amide an antagonist at the glucagon-like peptide 1-(7-36)-amide receptor of insulin-secreting beta-cells. J Biol Chem. 1993;268(26):19650–5.
Gulaboski R. Scholz FJTJoPCB. Lipophilicity of Peptide Anions: An Experimental Data Set for Lipophilicity Calculations. 2003;107(23):5650–7.
Meek JL. Prediction of peptide retention times in high-pressure liquid chromatography on the basis of amino acid composition. Proc Natl Acad Sci USA. 1980;77(3):1632–6.
Biswas KM, DeVido DR, Dorsey JG. Evaluation of methods for measuring amino acid hydrophobicities and interactions. J Chromatogr A. 2003;1000(1–2):637–55.
Livia VK, Gabriela IB, Paul B, Mark K, Dorothy KJA, Dmpk. Application of biomimetic HPLC to estimate lipophilicity, protein and phospholipid binding of potential peptide therapeutics. ADMET and DMPK. 2018;6(2):162–75.
K. V, Bevan C, Reynolds D. Chromatographic Hydrophobicity Index by Fast-Gradient RP-HPLC:PђS A High-Throughput Alternative to log P/log D. Analytic Chem. 1997;69(11):2022–9.
Berg S, Kärrberg L, Suljovic D, Seeliger F, Söderberg M, Perez-Alcazar M, Van Zuydam N, Abrahamsson B, Hugerth AM, Davies N, Bergström CAS. Impact of Intestinal Concentration and Colloidal Structure on the Permeation-Enhancing Efficiency of Sodium Caprate in the Rat. Mol Pharm. 2022;19(1):200–12.
Walsh EG, Adamczyk BE, Chalasani KB, Maher S, O’Toole EB, Fox JS, Leonard TW, Brayden DJ. Oral delivery of macromolecules: rationale underpinning Gastrointestinal Permeation Enhancement Technology (GIPET). Ther Deliv. 2011;2(12):1595–610.
Baekdal TA, Thomsen M, V. K, Hansen CW, Anderson TW. Pharmacokinetics, Safety, and Tolerability of Oral Semaglutide in Subjects With Hepatic Impairment. J Clin Pharmacol. 2018;58(10):1314–23.
Meier JJ, Granhall C, Hoevelmann U, Navarria A, Plum-Moerschel L, Ramesh C, Tannapfel A, Kapitza C. Effect of upper gastrointestinal disease on the pharmacokinetics of oral semaglutide in subjects with type 2 diabetes. Diabetes Obes Metab. 2022;24(4):684–92.
Ghai R, Falconer RJ, Collins BM. Applications of isothermal titration calorimetry in pure and applied research–survey of the literature from 2010. J Mol Recognit. 2012;25(1):32–52.
Frederiksen TM, Sønderby P, Ryberg LA, Harris P, Bukrinski JT, Scharff-Poulsen AM, Elf-Lind MN, Peters GH. Oligomerization of a Glucagon-like Peptide 1 Analog: Bridging Experiment and Simulations. Biophys J. 2015;109(6):1202–13.
Venanzi M, Savioli M, Cimino R, Gatto E, Palleschi A, Ripani G, Cicero D, Placidi E, Orvieto F, Bianchi E. A spectroscopic and molecular dynamics study on the aggregation process of a long-acting lipidated therapeutic peptide: the case of semaglutide. Soft Matter. 2020;16(44):10122–31.
Neidigh JW, Fesinmeyer RM, Prickett KS, Andersen NH. Exendin-4 and glucagon-like-peptide-1: NMR structural comparisons in the solution and micelle-associated states. Biochemistry. 2001;40(44):13188–200.
Lee JG, Ryu JH, Kim SM, Park MY, Kim SH, Shin YG, Sohn JW, Kim HH, Park ZY, Seong JY, Kim JI. Replacement of the C-terminal Trp-cage of exendin-4 with a fatty acid improves therapeutic utility. Biochem Pharmacol. 2018;151:59–68.
Pechenov S, Revell J, Will S, Naylor J, Tyagi P, Patel C, Liang L, Tseng L, Huang Y, Rosenbaum AI, Balic K, Konkar A, Grimsby J, Subramony JA. Development of an orally delivered GLP-1 receptor agonist through peptide engineering and drug delivery to treat chronic disease. Sci Rep. 2021;11(1):22521.
McCartney F, Gleeson JP, Brayden DJ. Safety concerns over the use of intestinal permeation enhancers: A mini-review. Tissue Barriers. 2016;4(2):e1176822.
Drucker DJ. Advances in oral peptide therapeutics. Nat Rev Drug Discov. 2020;19(4):277–89.
Halberg IB, Lyby K, Wassermann K, Heise T, Zijlstra E, Plum-Mörschel L. Efficacy and safety of oral basal insulin versus subcutaneous insulin glargine in type 2 diabetes: a randomised, double-blind, phase 2 trial. Lancet Diabetes Endocrinol. 2019;7(3):179–88.
Lee YH, Sinko PJ. Oral delivery of salmon calcitonin. Adv Drug Deliv Rev. 2000;42(3):225–38.
Twarog C, Fattah S, Heade J, Maher S, Fattal E, Brayden DJ. Intestinal Permeation Enhancers for Oral Delivery of Macromolecules: A Comparison between Salcaprozate Sodium (SNAC) and Sodium Caprate (C(10)). Pharmaceutics. 2019;11(2):78.
Alani AW, Robinson JR. Mechanistic understanding of oral drug absorption enhancement of cromolyn sodium by an amino acid derivative. Pharm Res. 2008;25(1):48–54.
Twarog C, McCartney F, Harrison SM, Illel B, Fattal E, Brayden DJ. Comparison of the effects of the intestinal permeation enhancers, SNAC and sodium caprate (C(10)): Isolated rat intestinal mucosae and sacs. Europ J Pharmaceutic Sci Official J Europ Federation Pharmaceutic Sci. 2021;158:105685.
Chen EP, Doan K, Portelli S, Coatney R, Vaden V, Wei SJPR. Gastric pH and Gastric Residence Time in Fasted and Fed Conscious Cynomolgus Monkeys Using the Bravo pH System. 2008;25(1):123–34.
McConnell EL, Basit AW, Murdan S. Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in-vivo experiments. J Pharm Pharmacol. 2008;60(1):63–70.
Franklin RA. The influence of gastric emptying on plasma concentrations of the analgesic, meptazinol. Br J Pharmacol. 1977;59(4):565–9.
Drugs@FDA: FDA-Approved Drugs. New Drug Application (NDA): 213051. In: administration USFad, editor. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/213051Orig1s000PharmR.pdf. Accessed 6 May 2021
ACKNOWLEDGEMENTS AND DISCLOSURES
Yadan Mao is gratefully thanked for correcting the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Hao Chen, Yun Lu, Shuai Shi, Qiang Zhang, Xiaoli Cao, Lei Sun, Dong An, Xiaojie Zhang, Xianglin Kong are employees of Jiangsu Hengrui Pharmaceuticals Co., Ltd.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, H., Lu, Y., Shi, S. et al. Design and Development of a New Glucagon-Like Peptide-1 Receptor Agonist to Obtain High Oral Bioavailability. Pharm Res 39, 1891–1906 (2022). https://doi.org/10.1007/s11095-022-03265-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03265-3