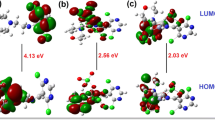
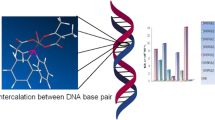
Ni(II) and Pt(II) complexes of tetrazole-containing Schiff bases were synthesized and characterized by physicochemical and various spectroscopic studies. To examine its potential as a candidate anticancer drug, the binding properties of ct-DNA were investigated. The characteristic binding constant (Kb) and binding mode of the complexes with calf thymus DNA (ct-DNA) were determined using absorption titration (1.99 – 76.71 × 104 M-1). According to the kinetic and thermodynamic parameters, the binding constant and spontaneity of the Pt(Tet-SalH) complex were found to be larger. The well-diffusion method was used to deduce the antibacterial potency of Schiff bases and their complexes. All these substances have been examined for antibacterial activity against pathogenic strains and antifungal activity. Only complex [Pt(3,5-Br-Sal-Tet)H2O]Cl∙H2O showed activity against all the microorganisms studied.








Similar content being viewed by others
References
S. Sayed, S. Dawood, S. Ibrahim, et al., Biointerface Res. Appl. Chem., 10(6), 6936 – 6963 (2020).
D. E. Reichert, J. S. Lewis, C. J. Anderson, Coord. Chem. Rev., 184(1), 3 – 66 (1999).
D. Nartop, P. Gürkan, N. Sarı, et al., J. Coord. Chem., 61(21), 3516 – 3524 (2007).
N. Sarı, N. Pişkin, H. Öğütcü, et al., Med. Chem. Res., 22(2), 580 – 587 (2013).
K. C. Emregül, E. Düzgün, O. Atakol, Corros. Sci., 48(10), 3243 – 3260 (2006).
J. Zhang, S.Wang, Y. Ba, et al., Eur. J. Med. Chem., 178, 341 – 351 (2019).
Y. Yıldırır, M. F. Us, N. Çolak, et al., Med. Chem. Res., 18(2), 91 – 97 (2009).
C.-X Wei, M. Bian and G.-H. Gong, Molecules., 20(4), 5528 – 5553 (2015).
G. I. Koldobskii, V. A. Ostrovskii and B. V. Gidaspov. Chem. Heterocycl. Compd., 16(7), 665 – 674 (1980).
S. Yavuz, O. Aydın, S. Çete, et al., Med. Chem. Res., 19(2), 120 – 126 (2010).
R. L. Siegel, K. D. Miller, A. Jemal, et al., Ca: Cancer J. Clin., 66(1), 7 – 30 (2016).
A. Di Lorenzo, M. T. Bedford, FEBS Lett., 585(13), 2024 – 2031 (2011).
A. R. Katritzky, C. A. Ramsden, E. F. V. Scriven, R. J. K. Taylor, Comprehensive Heterocyclic Chemistry III, Vol. 6: Other Five-membered Rings with Three or more Heteroatoms, and their Fused Carbocyclic Derivatives (2008), pp. 257 – 423.
Y. Tao, J. R. Li, Z. Chang, et al., Cryst. Growth Des., 10(2), 564 – 574 (2010).
N. Fischer, T. M. Klapötke, S. Scheutzow, et al., Cent. Eur. J. Energ. Mater., 5(3 – 4), 3 – 18 (2008).
J. Vanco, Z. Sindelar, Z. Dvorak, et al., J. Inorg. Biochem., 142, 92 – 100 (2015).
R. Bera, B. K. Sahoo, K. S. Ghosh, et al., Int. J. Biol. Macromol., 42(1), 14 – 21 (2008).
S. Niloufar, D. S. Farzad, B. Sima, et al., J. Biomol. Struct. Dyn., 37(2), 359 – 371 (2019).
D. Nartop, B. Demirel, M. Güleç, et al., J. Biochem. Mol. Toxicol., 34(2), e22432 (2019).
S. Koçoğlu, H. Öğütcü, Z. Hayvalý, Res. Chem. Intermed., 4(45), 2403 – 2427 (2019).
S. Rubab, S. Bahadur, U. Hanif, et al., Biocatal. Agric. Biotechnol., 31, 101894 (2021).
I. Sakıyan, E. Logoglu, S. Arslan, et al., Biometals, 17(2), 115 (2004).
A. A Popov, V. M. Senyavin, A. A. Granovsky, Fuller. Nanotub. Carbon Nanostruct., 12(1 – 2), 305 – 310 (2005).
D. Sinirliogglu, A. E. Müftüoglu, A. Bozkurt, J. Polym. Res., 20, 242 – 250 (2013).
R. M. Silverstein, G. C. Bassler, T. C. Morrill, Spectrometric identification of organic compounds, 4. Edition Jhon Wiley and Sons Inc USA (1981).
G. C. Percy, D. A. Thornton, J. Inorg. Nucl. Chem., 34(11), 3357 – 3367 (1972).
S. Yavuz, Y. Unal, O. Pamir, et al., Arch. Pharm., 34(6), 455 – 462 (2013).
Y. Yildirir, O. Pamir, S. Yavuz, et al., J. Heterocycl. Chem., 50(S1), E93 – E99 (2013).
E. H. Blackburn, Nature, 408, 53e56 (2000).
S. Artandi, S. L. Chang, S. Lee Alson, et al., Nature, 406, 641 – 645 (2000).
Y. Sun, Y. Lu, M. Bian, et al., Eur. J. Med. Chem., 211, 113098 – 113105 (2021).
T. C. Johnstone, K. Suntharalingam, S. J. Lippard, Chem. Rev., 116(5), 3436 – 3486 (2016).
J. Afzal, N. Ullah, Z. Hussain, et al., Matrix Science Pharma, 1(2), 17 – 19 (2017).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ulular, M., Sarı, N., Han, F. et al. Synthesis, Antimicrobial, and DNA-Binding Evaluation of Novel Schiff Bases Containing Tetrazole Moiety And Their Ni(II) and Pt(II) Complexes. Pharm Chem J 57, 1609–1620 (2024). https://doi.org/10.1007/s11094-024-03056-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-024-03056-7