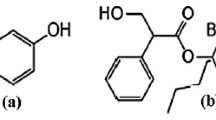
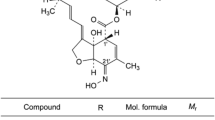
Quetiapine fumarate is an atypical antipsychotic drug, which is clinically used for the treatment of depression and bipolar disorders. A stability indicating method is required for the quality control of pharmaceutical dosage forms. This work focused on the developments of a rapid stability indicating reversed-phase HPLC-UV method for determination of quetiapine fumarate in tablets and extemporaneous formulations. Quetiapine fumarate was simultaneously determined in the presence of three impurities: quetiapine related compound B, quetiapine related compound G, and quetiapine N-oxide within 9 min. Optimal HPLC conditions were realized using Zorbax C8 column (100 mm × 4.6 cm i.d., 3 μm particle). Gradient elution utilized mobile composition of 0.15% triethylamine (pH 6.0) and acetonitrile : methanol (80:20) mixture at a flow rate of 1.2 mL/min with detection wavelength at 252 nm. The proposed method showed good linearity (r2 > 0.998), precision (RSDs ≤ 2.2), and accuracy (%recovery 96.8 – 99.4, RSD ≤ 1.9) with acceptable limits of detection (LOD ≤ 3.0 μg/mL) and quantitation (LOQ ≤ 10 μg/mL) at RSD ≤ 2.8). The method was successfully applied to the determination of quetiapine fumarate in pharmaceutical formulations and stability testing of extemporaneous formulations.




Similar content being viewed by others
References
N. H. Jensen, R. M. Rodriguiz, M. G. Caron, et al., Neuropsychopharmacology, 33(10), 2303 – 2312 (2008).
M. Jackson and A. Lowey, Handbook of Extemporaneous Preparations, Pharmaceutical Press: Illinois (2010).
J. F. Marriott, K. A. Wilson, C. A. Langley, and D. Belcher, Pharmaceutical Compounding and Dispensing, Pharmaceutical Press: London (2006).
B. D. Glass and A. Haywood, J. Pharm. Pharm. Sci., 9(3), 398 – 426 (2006).
United States Pharmacopeia - National Formulary, United States Pharmacopoeial Convention, Rockville, MD (2019).
British Pharmacopoeia, Commission British Pharmacopoeia, The Stationery Office: London (2019).
N. Rajendraprasad, B. Kanakapura, K. B. Vinay, Croat. Chem. Acta, 85(1), 9 – 17 (2012).
K. B. Vinay and H. D. Revenasiddappa, Chem. Ind. Chem. Eng. Q., 18(2), 263 – 272 (2012).
I. M. Mostafa, M. A. Omar, D. M. Nagy, et al., Spectrochim. Acta. A. Mol. Biomol. Spectrosc., 196, 196 – 201 (2018).
M. Ławrywianiec, J. Smajdor, B. Paczosa-Bator, et al., Anal. Meth., 9, 6662 – 6668 (2017).
R. K. Trivedi and M. C. Patel, Sci. Pharm., 79(1), 97 – 111 (2011).
M. Silva Gracia, A. Köppl, S. Unholzer, et al., Biomed. Chromatogr., 31(10), 1 – 11 (2017).
P. C. Pires Rosa, I. F. Rodrigues Pires, B. E. Ortega Markman, et al., J. Appl. Pharm. Sci., 3(8), 6 – 15 (2013).
E. Vessalli, L. Edjlali, M. Rezaei, et al., Asian J. Chem., 25(8), 4141 – 4144 (2013).
F. Belal, A. Elbrashy, M Eid, et al., J. Liq. Chromatogr. Rel. Tech., 31(9), 1283 – 1298 (2008).
N. Kumar, D. Sangeetha, R. Goyal, et al., Acta Chromatogr., 25(2), 393 – 409 (2013).
K. B. Venkata, S. R. Battula, S. Dubey, J. Chem., 2013, 1 – 8 (2013).
S. A. Bellomarino, A. J. Brown, X. A. Conlan, et al., Talanta, 77(5), 1873 – 1876 (2009).
M. Li, S. Zhang, A. Shi, et al., J. Chromatogr. B. Anal. Technol. Biomed. Life Sci., 1060, 10 – 14 (2017).
S. D. Dalvi, R. K. Nanda, S. S. Chitlange, Int. J. Pharm. Sci. Res., 9(7), 2875 – 2882 (2018).
M. Blessy, R. D. Patel, P. N. Prajapati, et al., J. Pharm. Anal., 4(3), 159 – 165 (2014).
N. A. Épshtein, Pharm. Chem. J., 53(12), 1174 – 1183 (2020).
ICH (Q2 R1), Validation of Analytical Procedures: Text and Methodology, in: Proceedings of International Conference on Harmonization, November (1996).
ICH (Q1A R2), Stability Testing of New Drug Substances and Products, in: Proceedings of International Conference on Harmonization, February (2003).
B. W. Rossiter and R. C. Baetzold (eds.), Physical Methods in Chemistry, Vol. 10, Supplement and Cumulative Index, Jonh Wiley & Sons: New York (1993).
Acknowledgements
This research project was financially supported by the Mahidol University (Grant no. 4044, Project no. 12319). The authors would like to thank Kenyaku Co. Ltd. (Bangkok, Thailand) for providing the standard quetiapine N-oxide used in this study.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Nuchtavorn, N., Leanpolchareanchai, J., Chanton, D. et al. A Rapid Stability Indicating HPLC Method for Determination of Quetiapine Fumarate in Tablets and Extemporaneous Formulations. Pharm Chem J 55, 845–854 (2021). https://doi.org/10.1007/s11094-021-02505-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-021-02505-x