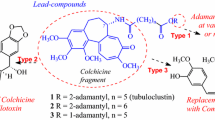
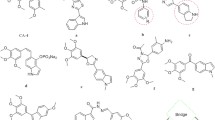
Combretastatin analogs of the antitumor agent tubuloclustin {N-[7-(adamant-2-yloxy)-7-oxoheptanoyl]-Ndeacetylcolchicine} were prepared via esterification of combretastatin by monoesters of pimelic or adipic acid with adamantan-2-ol or (adamantan-1-yl)methanol. These conjugates were stable and cytotoxic to human lung carcinoma A549 cells (EC50 ≈ 50 – 70 nM) and caused depolymerization of microtubules and slight clustering of tubulin. Tubuloclustin analogs with shortened linkers were prepared via amidation by N-deacetylcolchicine of monoesters of adipic or succinic acids with adamantan-1-ol or (adamantan-1-yl)methanol. The conjugate N-[6-(adamantyl)-6-oxohexanoyl]-N-deacetylcolchicine was more active (EC50 ≈ 4 nM) than tubuloclustin and promoted strong tubulin clusterization. All compounds induced apoptosis of A549 cells. Tests in vivo of N-[6-(adamantyl)-6-oxoheaxnoyl]-N-deacetylcolchicine on carcinoma A549 experimental models were concluded to be promising.





Similar content being viewed by others
References
B. Kumar, R. Kumar, I. Skvortsova, and V. Kumar, Curr. Cancer Drug Targets, 17(4), 357 – 375 (2017).
R. A. Stanton, K. M. Gernert, J. H. Nettles, and R. Aneja, Med. Res. Rev., 31(3), 443 – 481 (2011).
S. Wu, J. Fisher, J. Naci, et al., Chem. Res. Toxicol., 26(12), 1840 – 1861 (2013).
N. A. Zefirov and O. N. Zefirova, Zh. Org. Khim., 51(9), 1231 – 1240 (2015).
K. Nepali, S. Sharma, M. Sharma, et al., Eur. J. Med. Chem., 77, 422 – 487 (2014).
E. C. Breen and J. J. Walsh, Curr. Med. Chem., 17, 609 – 639 (2010).
C. Vilanova, S. Daaz-Oltra, J. Murga, et al., J. Med. Chem., 57(24), 10391 – 10403 (2014).
S. K. Kim, S. M. Cho, H. Kim, et al., Exp. Mol. Med., 45, E19 (2013).
P. Thomopoulou, J. Sachs, N. Teusch, et al., ACS Med. Chem. Lett., 7(2), 188 – 191 (2015).
O. N. Zefirova, H. Lemcke, M. Lantow, et al., ChemBioChem, 14(12), 1444 – 1449 (2013).
O. N. Zefirova, E. V. Nurieva, D. V. Shishov, et al., Bioorg. Med. Chem., 19(18), 5529 – 5538 (2011).
O. N. Zefirova, E. V. Nurieva, Ya. S. Glazkova, et al., Khim.-farm. Zh., 48, No. 6, 19 – 24 (2014); Pharm. Chem. J., 48(6), 373 – 378 (2014).
N. A. Zefirov, M. Hoppe, I. V. Kuznetsova, et al., Mendeleev Commun., 28(3), 225 – 344 (2018).
O. N. Zefirova, Ya. S. Glazkova, E. V. Nurieva, et al., Izv. Akad. Nauk, Ser. Khim., 5, 1126 – 1129 (2014).
O. N. Zefirova, E. V. Nurieva, B. Wobith, et al., Mol. Diversity, 21(3), 547 – 564 (2017).
E. V. Nurieva, N. A. Zefirov, A. V. Mamaeva, et al., Izv. Akad. Nauk, Ser. Khim., 4, 688 – 693 (2018).
B. Coggiola, F. Pagliai, G. Allegrone, et al., Bioorg. Med. Chem. Lett., 15(15), 3551 – 3554 (2005).
L. Lebeau, P. Ducray, and C. Mioskowski, Synth. Commun., 27(2), 293 – 296 (1997).
J. D. Bagnato, A. L. Eilers, R. A. Horton, and C. B. Grissom, J. Org. Chem., 69(26), 8987 (2004).
T. Mosmann, J. Immunol. Methods, 65(1 – 2), 55 – 63 (1983).
Acknowledgments
The work was financially supported by RFBR Grant 18–33–01121 mol a. We thank German Academic Exchange Service DAAD for supporting an academic exchange under terms of a collaboration agreement between Moscow and Rostock Universities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 53, No. 5, pp. 13 – 19, May, 2019.
Rights and permissions
About this article
Cite this article
Zefirov, N.A., Evteeva, Y.A., Fatkulin, A.R. et al. Synthesis, Antiproliferative Activity, and Effect on Carcinoma A549 Cell Microtubules of New Tubuloclustin Analogs. Pharm Chem J 53, 423–428 (2019). https://doi.org/10.1007/s11094-019-02014-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-019-02014-y