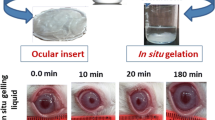
The present study aims to prepare ocular inserts of cetirizine hydrochloride, an antihistamine used to treat allergic conjunctivitis, allergic rhinitis, uveitis and urticaria. Solvent casting method was used to prepare inserts of hydroxypropyl methylcellulose (HPMC) and polyvinyl alcohol (PVA) as film forming polymers, and polyethylene glycol (PG) and glycerin were used as plasticizers. Various physicochemical parameters of six ocular insert compositions were evaluated, including swelling behavior and in vitro drug release and kinetics. Formulation F4 showed results of in vitro studies in most appropriate range, with sustained drug release (99.12% at 210 min) following non-fickian diffusion. Drug – polymer compatibility study verified no chemical interaction between the drug and polymer. Therefore, formulation F4 was selected for in vivo studies in albino rabbits. The values of Cmax, AUC, and Tmax of formulation F4 were 478.70 ng/mL, 3013.58 ng/(mL h), and 2.3 h, respectively. Ocular inserts of cetirizine hydrochloride provide a valuable alternative for the treatment of ocular inflammatory diseases as these formulations ensure sustained drug release and increased residence time.




Similar content being viewed by others
References
M. H. Aburahma and A. A. Mahmoud, AAPS PharmSciTech, 12, 1334 – 1347 (2011).
K. P. S. Kumar, D. Bhowmik, G. Harish, et al., The Pharma Innovation., 1 (2013)
S. El-Sousi, A. Nacher, C. Mura, et al., J. Pharm. Pharmacol., 65, 193 – 200 (2012).
A. Kumari, P. K. Sharma, K. Vipin, et al., J. Adv. Pharm. Technol. Res., 1, 291 – 296 (2010).
N. L. Eremeev, V. N. Ermakova, O. A. Kost, et al., Pharm. Chem. J., 40, 36 – 39 (2006).
S. Ramkanth, C. M. Chetty, M. Alagusundaram, et al., Int. J. Pharm. Tech. Res., 1, 1219 – 1223 (2009).
M. K. Arýcý, D. S. Arýcý, A. Topalkara, et al., Clin. Exp. Ophthalmol., 28, 113 – 117 (2000).
A. Zimmer and J. Kreuter, Adv. Drug Deliv. Rev., 16, 61 – 73 (1995).
A. Durrani, S. Farr, and I. Kellaway, J. Pharm. Pharmacol., 47, 581 – 584 (1995).
I. Genta, B. Conti, P. Perugini, et al., J. Pharm. Pharmacol., 49, 737 – 742 (1997).
P. Giunchedi, U. Conte, P. Chetoni, et al., Eur. J. Pharm. Sci., 9, 1 – 7 (1999).
S. F. Bernatchez, A. Merkli, T. L. Minh, et al., J. Biomed. Mater. Res., 28, 1037 – 1046 (1994).
R. Gurny, T. Boye, and H. Ibrahim, J. Control. Release, 2, 353 – 361 (1985).
K. Langer, A. Zimmer, and J. Kreuter, STP Pharma. Sci., 7, 445 – 451 (1997).
M. S. Nagarsenker, V. Y. Londhe, and G. D. Nadkarni, Int. J. Pharm., 190, 63 – 71 (1999).
S. L. Law, K. J. Huang, and C. H. Chiang, J. Control. Release, 63, 135 – 140 (2000).
E. Bertelmann, T. Ritter, K. Vogt, et al., Ophthalmic Res., 35, 117 – 124 (2003).
F. Gurtler and R. Gurny, Drug Dev. Ind. Pharm., 21, 1 – 18 (1995).
M. F. Saettone and L. Salminen, Adv. Drug Deliv. Rev., 16, 95 – 106 (1995).
F. Gurtler, V. Kaltsatos, B. Boisrame, et al., J. Control. Release., 33, 231 – 236 (1995).
J. G. Souza, K. Dias, T. A. Pereira, et al., J. Pharm. Pharmacol., 66, 507 – 530 (2013).
M. J. Alonso and A. Sanchez, J. Pharm. Pharmacol., 55, 1451 – 1463 (2003).
Y. C. Lee, J. W. Millard, G. J. Negvesky, et al., Int. J. Pharm., 182, 121 – 126 (1999).
S. Kawakami, K. Nishida, T. Mukai, et al., J. Control. Release, 76, 255 – 263 (2001).
J. Ceulemans, A. Vermeire, E. Adriaens, et al., J. Control. Release, 77, 333 – 344 (2001).
P. Pawar, R. Katara, and D. Majumdar, Acta Pharm., 62, 93 – 104 (2012).
J. Balasubramaniam, A. Srinatha, G. Nath, et al., Indian J. Pharm. Sci., 68, 626 – 630 (2006).
Acknowledgments
The authors greatly appreciate the moral support of our research group.
DECLARATIONS
ᅟ
Conflict of Interest
The authors declare that they have no conlicts of interest to disclose.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shah, S.N.H., Nawaz, A., Javed, H. et al. Preparation and In Vitro/In Vivo Evaluation of Antihistaminic Ocular Inserts. Pharm Chem J 52, 615–622 (2018). https://doi.org/10.1007/s11094-018-1870-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-018-1870-x