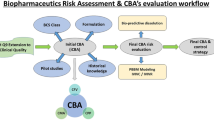
The urgency to satisfy pharmaceutical regulatory requirements, including drug quality risk management, prompted the application of risk assessment methodology during the pharmaceutical development (PD) stage of an actual drug production process to be studied. Risk assessment results for the industrial process in combination with information regarding risks to the final product that were obtained during the PD allowed an objective opinion about the influence of the product properties and process parameters on critical drug quality parameters during its mass production to be formulated.

Similar content being viewed by others
References
Guideline ST-N MOZU 42-3.0:2011. Drugs. Pharmaceutical Development [in Ukrainian] (ICH Q8), MOZU, Kyiv (2011).
Guideline ST-N MOZU 42-4.2:2011. Drugs. Quality Risk Management [in Ukrainian] (ICH Q9), MOZU, Kyiv (2011).
Guideline ST-N MOZU 42-4.0:2013. Drugs. Good Manufacturing Practice [in Ukrainian], MOZU, Kyiv (2013).
S. N. Kashutskii, S. V. Rusanova, and S. I. Dikhtyarev, Farmakom, No. 3, 54 – 62 (2013).
S. N. Kashutskii, S. V. Rusanova, S. I. Dikhtyarev, et al., in: Proceedings of the IVth All-Russian Scientific-Practical Conference with International Participation [in Russian], Vladikavkaz (2014), pp. 106 – 109.
S. N. Kashutskii, S. V. Rusanova, S. I. Dikhtyarev, et al., in: Proceedings of the IVth All-Russian Scientific-Practical Conference with International Participation [in Russian], Vladikavkaz (2014), pp. 110 – 112.
GOST R 51901.12-2007 [IEC 60812:2006, Risk management.
Procedure for failure mode and effects analysis (FMEA), MOD], Moscow (2008).
GOST R ISO / IEC 31010-2011 (ISO / IEC 31010:2009, Risk management. Risk assessment methods, IDT), Moscow (2012).
Author information
Authors and Affiliations
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 49, No. 11, pp. 49 – 53, November, 2015.
Rights and permissions
About this article
Cite this article
Kashutskii, S.N., Rusanova, S.V., Dikhtyarev, S.I. et al. Some Aspects of Quality Risk Management for the Fenspiride Hydrochloride (0.08 G Coated Tablets) Production Process at the Pharmaceutical Development Stage. Pharm Chem J 49, 771–775 (2016). https://doi.org/10.1007/s11094-016-1368-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-016-1368-3