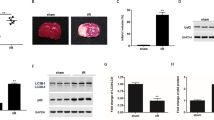
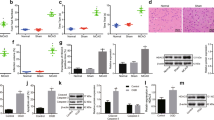
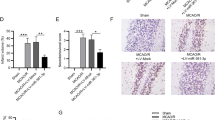
Abstract
The objective of this research was to investigate the role of lncRNA MALAT1 and HSP90 in the regulation of neuronal necroptosis in mice with cerebral ischemia-reperfusion (CIR). We used male C57BL/6J mice to establish a middle cerebral artery occlusion (MCAO) model and conducted in vitro experiments using the HT-22 mouse hippocampal neuron cell line. The cellular localization of NeuN and MLKL, as well as the expression levels of neuronal necroptosis factors, MALAT1, and HSP90 were analyzed. Cell viability and necroptosis were assessed, and we also investigated the relationship between MALAT1 and HSP90. The results showed that MALAT1 expression increased after MCAO and oxygen-glucose deprivation/re-oxygenation (OGD/R) treatment in both cerebral tissues and cells compared with the control group. The levels of neuronal necroptosis factors and the co-localization of NeuN and MLKL were also increased in MCAO mice compared with the Sham group. MALAT1 was found to interact with HSP90, and inhibition of HSP90 expression led to decreased phosphorylation levels of neuronal necroptosis factors. Inhibition of MALAT1 expression resulted in decreased co-localization levels of NeuN and MLKL, decreased phosphorylation levels of neuronal necroptosis factors, and reduced necroptosis rate in cerebral tissues. Furthermore, inhibiting MALAT1 expression also led to a shorter half-life of HSP90, increased ubiquitination level, and decreased phosphorylation levels of neuronal necroptosis factors in cells. In conclusion, this study demonstrated that lncRNA MALAT1 promotes neuronal necroptosis in CIR mice by stabilizing HSP90.






Similar content being viewed by others
Data Availability
All data are included in the text. If necessary, the original data can be obtained from the corresponding authors.
References
Saini V, Guada L, Yavagal DR (2021) Global epidemiology of Stroke and Access to acute ischemic stroke interventions. Neurology 97:S6–s16. https://doi.org/10.1212/wnl.0000000000012781
Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, Wang L, Jiang Y, Li Y, Wang Y, Chen Z, Wu S, Zhang Y, Wang D, Wang Y, Feigin VL (2017) Prevalence, incidence, and mortality of stroke in China: results from a Nationwide Population-Based survey of 480 687 adults. Circulation 135:759–771. https://doi.org/10.1161/circulationaha.116.025250
Barthels D, Das H (2020) Current advances in ischemic stroke research and therapies. Biochimica et biophysica acta. Mol basis disease 1866:165260. https://doi.org/10.1016/j.bbadis.2018.09.012
Simonsen CZ, Leslie-Mazwi TM, Thomalla G (2021) Which Imaging Approach should be used for stroke of unknown time of Onset? Stroke 52:373–380. https://doi.org/10.1161/strokeaha.120.032020
Leech T, Chattipakorn N, Chattipakorn SC (2019) The beneficial roles of metformin on the brain with cerebral ischaemia/reperfusion injury. Pharmacol Res 146:104261. https://doi.org/10.1016/j.phrs.2019.104261
Kelmanson IV, Shokhina AG, Kotova DA, Pochechuev MS, Ivanova AD, Kostyuk AI, Panova AS, Borodinova AA, Solotenkov MA, Stepanov EA, Raevskii RI, Moshchenko AA, Pak VV, Ermakova YG, van Belle GJC, Tarabykin V, Balaban PM, Fedotov IV, Fedotov AB, Conrad M, Bogeski I, Katschinski DM, Doeppner TR, Bähr M, Zheltikov AM, Belousov VV, Bilan DS (2021) In vivo dynamics of acidosis and oxidative stress in the acute phase of an ischemic stroke in a rodent model. Redox Biol 48:102178. https://doi.org/10.1016/j.redox.2021.102178
Mohamed SK, Ahmed AAE, El Morsy EM, Nofal S (2019) The protective effect of zeranol in cerebral ischemia reperfusion via p-CREB overexpression. Life Sci 217:212–221. https://doi.org/10.1016/j.lfs.2018.12.017
Movahed M, Brockie S, Hong J, Fehlings MG (2021) Transcriptomic Hallmarks of Ischemia-Reperfusion Injury. https://doi.org/10.3390/cells10071838. Cells 10
Naito MG, Xu D, Amin P, Lee J, Wang H, Li W, Kelliher M, Pasparakis M, Yuan J (2020) Sequential activation of necroptosis and apoptosis cooperates to mediate vascular and neural pathology in stroke. Proc Natl Acad Sci U S A 117:4959–4970. https://doi.org/10.1073/pnas.1916427117
Horne CR, Samson AL, Murphy JM (2022) The web of death: the expanding complexity of necroptotic signaling. Trends Cell Biol. https://doi.org/10.1016/j.tcb.2022.05.008
Xu Y, Wang J, Song X, Wei R, He F, Peng G, Luo B (2016) Protective mechanisms of CA074-me (other than cathepsin-B inhibition) against programmed necrosis induced by global cerebral ischemia/reperfusion injury in rats. Brain Res Bull 120:97–105. https://doi.org/10.1016/j.brainresbull.2015.11.007
Chen W, Teng X, Ding H, Xie Z, Cheng P, Liu Z, Feng T, Zhang X, Huang W, Geng D (2022) Nrf2 protects against cerebral ischemia-reperfusion injury by suppressing programmed necrosis and inflammatory signaling pathways. Ann Transl Med 10:285. https://doi.org/10.21037/atm-22-604
Schopf FH, Biebl MM, Buchner J (2017) The HSP90 chaperone machinery. Nat Rev Mol Cell Biol 18:345–360. https://doi.org/10.1038/nrm.2017.20
Hoter A, El-Sabban ME, Naim HY (2018) The HSP90 Family: structure, regulation, function, and implications in Health and Disease. Int J Mol Sci 19. https://doi.org/10.3390/ijms19092560
Szyller J, Bil-Lula I (2021) Heat Shock Proteins in Oxidative Stress and Ischemia/Reperfusion Injury and Benefits from Physical Exercises: A Review to the Current Knowledge. Oxid Med Cell Longev 2021:6678457. https://doi.org/10.1155/2021/6678457
Wang Y, Ma H, Huang J, Yao Z, Yu J, Zhang W, Zhang L, Wang Z, Zhuang C (2021) Discovery of bardoxolone derivatives as novel orally active necroptosis inhibitors. Eur J Med Chem 212:113030. https://doi.org/10.1016/j.ejmech.2020.113030
Yu X, Mao M, Liu X, Shen T, Li T, Yu H, Zhang J, Chen X, Zhao X, Zhu D (2020) A cytosolic heat shock protein 90 and co-chaperone p23 complex activates RIPK3/MLKL during necroptosis of endothelial cells in acute respiratory distress syndrome. J Mol Med (Berl) 98:569–583. https://doi.org/10.1007/s00109-020-01886-y
Jacobsen AV, Lowes KN, Tanzer MC, Lucet IS, Hildebrand JM, Petrie EJ, van Delft MF, Liu Z, Conos SA, Zhang JG, Huang DC, Silke J, Lessene G, Murphy JM (2016) HSP90 activity is required for MLKL oligomerisation and membrane translocation and the induction of necroptotic cell death. Cell Death Dis 7:e2051. https://doi.org/10.1038/cddis.2015.386
Li D, Xu T, Cao Y, Wang H, Li L, Chen S, Wang X, Shen Z (2015) A cytosolic heat shock protein 90 and cochaperone CDC37 complex is required for RIP3 activation during necroptosis. Proc Natl Acad Sci U S A 112:5017–5022. https://doi.org/10.1073/pnas.1505244112
Wang Z, Guo LM, Wang Y, Zhou HK, Wang SC, Chen D, Huang JF, Xiong K (2018) Inhibition of HSP90α protects cultured neurons from oxygen-glucose deprivation induced necroptosis by decreasing RIP3 expression. J Cell Physiol 233:4864–4884. https://doi.org/10.1002/jcp.26294
Chen WW, Yu H, Fan HB, Zhang CC, Zhang M, Zhang C, Cheng Y, Kong J, Liu CF, Geng D, Xu X (2012) RIP1 mediates the protection of geldanamycin on neuronal injury induced by oxygen-glucose deprivation combined with zVAD in primary cortical neurons. J Neurochem 120:70–77. https://doi.org/10.1111/j.1471-4159.2011.07526.x
Jiang N, Zhang X, Gu X, Li X, Shang L (2021) Progress in understanding the role of lncRNA in programmed cell death. Cell Death Discov 7:30. https://doi.org/10.1038/s41420-021-00407-1
Liu W, Wang Z, Liu L, Yang Z, Liu S, Ma Z, Liu Y, Ma Y, Zhang L, Zhang X, Jiang M, Cao X (2020) LncRNA Malat1 inhibition of TDP43 cleavage suppresses IRF3-initiated antiviral innate immunity. Proc Natl Acad Sci U S A 117:23695–23706. https://doi.org/10.1073/pnas.2003932117
Ji P, Diederichs S, Wang W, Böing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, Thomas M, Berdel WE, Serve H, Müller-Tidow C (2003) MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene 22:8031–8041. https://doi.org/10.1038/sj.onc.1206928
Huang XJ, Xia Y, He GF, Zheng LL, Cai YP, Yin Y, Wu Q (2018) MALAT1 promotes angiogenesis of breast cancer. Oncol Rep 40:2683–2689. https://doi.org/10.3892/or.2018.6705
Lee NK, Lee JH, Ivan C, Ling H, Zhang X, Park CH, Calin GA, Lee SK (2017) MALAT1 promoted invasiveness of gastric adenocarcinoma. BMC Cancer 17:46. https://doi.org/10.1186/s12885-016-2988-4
Jen J, Tang YA, Lu YH, Lin CC, Lai WW, Wang YC (2017) Oct4 transcriptionally regulates the expression of long non-coding RNAs NEAT1 and MALAT1 to promote lung cancer progression. Mol Cancer 16:104. https://doi.org/10.1186/s12943-017-0674-z
Zhang X, Tang X, Liu K, Hamblin MH, Yin KJ (2017) Long noncoding RNA Malat1 regulates cerebrovascular pathologies in ischemic stroke. J Neurosci 37:1797–1806. https://doi.org/10.1523/jneurosci.3389-16.2017
Wang C, Qu Y, Suo R, Zhu Y (2019) Long non-coding RNA MALAT1 regulates angiogenesis following oxygen-glucose deprivation/reoxygenation. J Cell Mol Med 23:2970–2983. https://doi.org/10.1111/jcmm.14204
Wang H, Zheng X, Jin J, Zheng L, Guan T, Huo Y, Xie S, Wu Y, Chen W (2020) LncRNA MALAT1 silencing protects against cerebral ischemia-reperfusion injury through miR-145 to regulate AQP4. J Biomed Sci 27:40. https://doi.org/10.1186/s12929-020-00635-0
Tan X, Guo W, Peng Z, Gu C, Xiang P, Tu Y, Fei H, Liu X, Lu Y, Li M, Wang H, Luo Y, Yang J (2021) LncRNA-Malat1 down-regulates mir-211-5p expression to promote neuronal damage from cerebral ischemia reperfusion injury. Biochem Pharmacol 192:114694. https://doi.org/10.1016/j.bcp.2021.114694
Jia Y, Yi L, Li Q, Liu T, Yang S (2021) LncRNA MALAT1 aggravates oxygen-glucose deprivation/reoxygenation-induced neuronal endoplasmic reticulum stress and apoptosis via the miR-195a-5p/HMGA1 axis. Biol Res 54:8. https://doi.org/10.1186/s40659-021-00331-9
Shu J, Yang L, Wei W, Zhang L (2022) Identification of programmed cell death-related gene signature and associated regulatory axis in cerebral ischemia/reperfusion injury. Front Genet 13:934154. https://doi.org/10.3389/fgene.2022.934154
Liang J, Wang Q, Li JQ, Guo T, Yu D (2020) Long non-coding RNA MEG3 promotes cerebral ischemia-reperfusion injury through increasing pyroptosis by targeting miR-485/AIM2 axis. Exp Neurol 325:113139. https://doi.org/10.1016/j.expneurol.2019.113139
Li L, Dong L, Xiao Z, He W, Zhao J, Pan H, Chu B, Cheng J, Wang H (2020) Integrated analysis of the proteome and transcriptome in a MCAO mouse model revealed the molecular landscape during stroke progression. J Adv Res 24:13–27. https://doi.org/10.1016/j.jare.2020.01.005
Cao DW, Liu MM, Duan R, Tao YF, Zhou JS, Fang WR, Zhu JR, Niu L, Sun JG (2020) The lncRNA Malat1 functions as a ceRNA to contribute to berberine-mediated inhibition of HMGB1 by sponging miR-181c-5p in poststroke inflammation. Acta Pharmacol Sin 41:22–33. https://doi.org/10.1038/s41401-019-0284-y
Wang YL, Lin CH, Chen CC, Chang CP, Lin KC, Su FC, Chou W (2019) Exercise Preconditioning attenuates neurological Injury by Preserving Old and newly formed HSP72-Containing neurons in focal brain ischemia rats. Int J Med Sci 16:675–685. https://doi.org/10.7150/ijms.32962
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91. https://doi.org/10.1161/01.str.20.1.84
Liao Y, Cheng J, Kong X, Li S, Li X, Zhang M, Zhang H, Yang T, Dong Y, Li J, Xu Y, Yuan Z (2020) HDAC3 inhibition ameliorates ischemia/reperfusion-induced brain injury by regulating the microglial cGAS-STING pathway. Theranostics 10:9644–9662. https://doi.org/10.7150/thno.47651
Harhouri K, Navarro C, Depetris D, Mattei MG, Nissan X, Cau P, De Sandre-Giovannoli A, Lévy N (2017) MG132-induced progerin clearance is mediated by autophagy activation and splicing regulation. EMBO Mol Med 9:1294–1313. https://doi.org/10.15252/emmm.201607315
Xie M, Yu T, Jing X, Ma L, Fan Y, Yang F, Ma P, Jiang H, Wu X, Shu Y, Xu T (2020) Exosomal circSHKBP1 promotes gastric cancer progression via regulating the miR-582-3p/HUR/VEGF axis and suppressing HSP90 degradation. Mol Cancer 19:112. https://doi.org/10.1186/s12943-020-01208-3
Yang X, Zhang S, He C, Xue P, Zhang L, He Z, Zang L, Feng B, Sun J, Zheng M (2020) METTL14 suppresses proliferation and metastasis of colorectal cancer by down-regulating oncogenic long non-coding RNA XIST. Mol Cancer 19:46. https://doi.org/10.1186/s12943-020-1146-4
Zhang X, Wang S, Wang H, Cao J, Huang X, Chen Z, Xu P, Sun G, Xu J, Lv J, Xu Z (2019) Circular RNA circNRIP1 acts as a microRNA-149-5p sponge to promote gastric cancer progression via the AKT1/mTOR pathway. Mol Cancer 18:20. https://doi.org/10.1186/s12943-018-0935-5
Nikseresht S, Khodagholi F, Ahmadiani A (2019) Protective effects of ex-527 on cerebral ischemia-reperfusion injury through necroptosis signaling pathway attenuation. J Cell Physiol 234:1816–1826. https://doi.org/10.1002/jcp.27055
Yang Y, He B, Zhang X, Yang R, Xia X, Chen L, Li R, Shen Z, Chen P (2022) Geraniin Protects against Cerebral Ischemia/Reperfusion Injury by Suppressing Oxidative Stress and Neuronal Apoptosis via Regulation of the Nrf2/HO-1 Pathway. Oxid Med Cell Longev 2022:2152746. https://doi.org/10.1155/2022/2152746
Ma HF, Zheng F, Su LJ, Zhang DW, Liu YN, Li F, Zhang YY, Gong SS, Kou JP (2022) Metabolomic profiling of Brain Protective Effect of Edaravone on Cerebral Ischemia-Reperfusion Injury in mice. Front Pharmacol 13:814942. https://doi.org/10.3389/fphar.2022.814942
Marunouchi T, Nishiumi C, Iinuma S, Yano E, Tanonaka K (2021) Effects of Hsp90 inhibitor on the RIP1-RIP3-MLKL pathway during the development of heart failure in mice. Eur J Pharmacol 898:173987. https://doi.org/10.1016/j.ejphar.2021.173987
Liu XQ, Liu MM, Jiang L, Gao L, Zhang Y, Huang YB, Wang X, Zhu W, Zeng HX, Meng XM, Wu YG (2022) A novel small molecule Hsp90 inhibitor, C-316-1, attenuates acute kidney injury by suppressing RIPK1-mediated inflammation and necroptosis. Int Immunopharmacol 108:108849. https://doi.org/10.1016/j.intimp.2022.108849
Heckmann BL, Tummers B, Green DR (2019) Crashing the computer: apoptosis vs. necroptosis in neuroinflammation. Cell Death Differ 26:41–52. https://doi.org/10.1038/s41418-018-0195-3
Fontecha-Barriuso M, Martín-Sanchez D, Martinez-Moreno JM, Cardenas-Villacres D, Carrasco S, Sanchez-Niño MD, Ruiz-Ortega M, Ortiz A, Sanz AB (2020) Molecular pathways driving omeprazole nephrotoxicity. Redox Biol 32:101464. https://doi.org/10.1016/j.redox.2020.101464
Chai SC, Davis K, Zhang Z, Zha L, Kirschner KF (2019) Effects of Tart Cherry juice on biomarkers of inflammation and oxidative stress in older adults. https://doi.org/10.3390/nu11020228. Nutrients 11
Zou Y, Tang Y, Fan W, Liu L, Jiao Y (2022) Cognition impairment of rat in undersea environment. Int J Environ Health Res 32:829–839. https://doi.org/10.1080/09603123.2020.1799955
Duan X, Liu X, Liu N, Huang Y, Jin Z, Zhang S, Ming Z, Chen H (2020) Inhibition of keratinocyte necroptosis mediated by RIPK1/RIPK3/MLKL provides a protective effect against psoriatic inflammation. Cell Death Dis 11:134. https://doi.org/10.1038/s41419-020-2328-0
Shi YL, Wang Q, Wei JC (2019) Influence of lncRNA-MALAT1 on neuronal apoptosis in rats with cerebral infarction through regulating the ERK/MAPK signaling pathway. Eur Rev Med Pharmacol Sci 23:8039–8048. https://doi.org/10.26355/eurrev_201909_19020
Somogyvári M, Khatatneh S, Sőti C (2022) Hsp90: from Cellular to Organismal Proteostasis. Cells 11. https://doi.org/10.3390/cells11162479
Qi X, Zhang X, Liu X, Tang W, Dai J, Chen A, Lin Q, Zhu T, Li J (2021) HDN-1 induces cell differentiation toward apoptosis in promyelocytic leukemia cells depending on its selective effect on client proteins of Hsp90. Toxicol Appl Pharmacol 417:115459. https://doi.org/10.1016/j.taap.2021.115459
Zhao XM, Chen Z, Zhao JB, Zhang PP, Pu YF, Jiang SH, Hou JJ, Cui YM, Jia XL, Zhang SQ (2016) Hsp90 modulates the stability of MLKL and is required for TNF-induced necroptosis. Cell Death Dis 7:e2089. https://doi.org/10.1038/cddis.2015.390
Yuan Z, Yi-Yun S, Hai-Yan Y (2020) Triad3A displays a critical role in suppression of cerebral ischemic/reperfusion (I/R) injury by regulating necroptosis. Biomed pharmacotherapy = Biomedecine pharmacotherapie 128:110045. https://doi.org/10.1016/j.biopha.2020.110045
Chen L, Fan H, Chu H, Du F, Chen Y, Hu L, Li Z, Wang W, Hou X, Yang L (2022) The HSP90 inhibitor 17-DMAG alleviates primary biliary cholangitis via cholangiocyte necroptosis prevention. J Cell Biochem 123:1857–1872. https://doi.org/10.1002/jcb.30321
Funding
The work was supported by Hainan Provincial Natural Science Foundation of China (Grant Number: 2019RC386) and Hainan Clinical Research Center for Cerebrovascular disease.
Author information
Authors and Affiliations
Contributions
“Shan Huang, Guoshuai Yang, and Dan Yu contributed to the study conception and design. Material preparation, data collection were performed by Shan Huang, Dan Hou, Lei Zhang, Chaoying Pei, and Ji Liang. Data analysis was performed by Junqi Li and Guoshuai Yang. The first draft of the manuscript was written by Shan Huang. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.“
Corresponding authors
Ethics declarations
Ethics approval
This study was approved by the Animal Ethics Committee of Haikou Affiliated Hospital of Central South University Xiangya School of Medicine (Approval number: 2022-058) and performed in accordance with the guidelines of the animal care and ethics committee.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
11064_2023_3991_MOESM2_ESM.jpg
Supplementary Figure S2. The expression of MLKL protein in cerebral tissues was detected using WB. *P < 0.05 vs. Sham. t-test.
11064_2023_3991_MOESM3_ESM.jpg
Supplementary Figure S3. Supplementary experiments of Fig. 2D and E. (A) The changes of MALAT1 level in cells in Fig. 2D were detected using RT-qPCR in the control group (OGD/R-0 h) and the OGD/R group (OGD/R-48 h). (B) WB was used to detect RIPK1, p-RIPK1, RIPK3, p-RIPK3, MLKL, and p-MLKL levels in the control group (OGD/R-0 h) and OGD/R group (OGD/R-48 h) in cells of Fig. 2E. *P < 0.05 vs. control. #P < 0.05 vs.sh-NC. one-way ANOVA.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, S., Hou, D., Zhang, L. et al. LncRNA MALAT1 Promoted Neuronal Necroptosis in Cerebral Ischemia-reperfusion Mice by Stabilizing HSP90. Neurochem Res 48, 3457–3471 (2023). https://doi.org/10.1007/s11064-023-03991-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-03991-z