Abstract
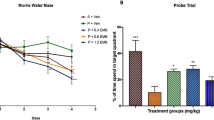
We aimed to investigate the probable protective effects of gallic acid (GA) on cognitive deficits, hippocampal long term potentiation (LTP) impairments, and molecular changes induced by cerebral ischemia/reperfusion (I/R) in rats following exposure to ambient dust storm. After pretreatment with GA (100 mg/kg), or vehicle (Veh) (normal saline, 2 ml/kg) for ten days, and 60 minutes’ exposure to dust storm including PM (PM, 2000–8000 g/m3) every day, 4-vessel occlusion (4VO) type of I/R was induced. Three days after I/R induction, we evaluated behavioral, electrophysiological, histopathological, molecular and brain tissue inflammatory cytokine changes. Our findings indicated that pretreatment with GA significantly reduced cognitive impairments caused by I/R (P < 0.05) and hippocampal LTP impairments caused by I/R after PM exposure (P < 0.001). Additionally, after exposure to PM, I/R significantly elevated the tumor necrosis factor α content (P < 0.01) and miR-124 level (P < 0.001) while pre-treatment with GA reduced the level of miR-124 (P < 0.001). Histopathological results also revealed that I/R and PM caused cell death in the hippocampus CA1 area (P < 0.001) and that GA decreased the rate of cell death (P < 0.001). Our findings show that GA can prevent brain inflammation, and thus cognitive and LTP deficits caused by I/R, PM exposure, or both.








Similar content being viewed by others
References
Kurosaki Y, Mikami M (2003) Recent frequent dust events and their relation to surface wind in East Asia. Geophys Res Lett. https://doi.org/10.1029/2003GL017261
Wang X, Bi X, Sheng G, Fu J (2006) Chemical composition and sources of PM10 and PM2. 5 aerosols in Guangzhou, China. Environ Monit Assess 119(1–3):425–439
Anuforom AC (2007) Spatial distribution and temporal variability of Harmattan dust haze in sub-sahel West Africa. Atmos Environ 41(39):9079–9090
Kellogg CA, Griffin DW, Garrison VH, Peak KK, Royall N, Smith RR et al (2004) Characterization of aerosolized bacteria and fungi from desert dust events in Mali, West Africa. Aerobiologia 20(2):99–110
Astort F, Sittner M, Ferraro SA, Orona NS, Maglione GA, De la Hoz A et al (2014) Pulmonary inflammation and cell death in mice after acute exposure to air particulate matter from an industrial region of Buenos Aires. Arch Environ Contam Toxicol 67(1):87–96
Davel AP, Lemos M, Pastro LM, Pedro SC, de André PA, Hebeda C et al (2012) Endothelial dysfunction in the pulmonary artery induced by concentrated fine particulate matter exposure is associated with local but not systemic inflammation. Toxicology 295(1–3):39–46
Cui Y, Sun Q, Liu Z (2016) Ambient particulate matter exposure and cardiovascular diseases: a focus on progenitor and stem cells. J Cell Mol Med 20(5):782–793
Weinmayr G, Hennig F, Fuks K, Nonnemacher M, Jakobs H, Möhlenkamp S et al (2015) Long-term exposure to fine particulate matter and incidence of type 2 diabetes mellitus in a cohort study: effects of total and traffic-specific air pollution. Environ Health 14(1):1–8
Jahangiri HM, Sarkaki A, Farbood Y, Dianat M, Goudarzi G (2020) Gallic acid affects blood-brain barrier permeability, behaviors, hippocampus local EEG, and brain oxidative stress in ischemic rats exposed to dusty particulate matter. Environ Sci Pollut Res 27(5):5281–5292
Xu X, Ha SU, Basnet R (2016) A review of epidemiological research on adverse neurological effects of exposure to ambient air pollution. Front public health 4:157
Schmid O, Möller W, Semmler-Behnke M, Ferron A, Karg G, Lipka E (2009) Dosimetry and toxicology of inhaled ultrafine particles. Biomarkers 14(sup1):67–73
Furuyama A, Kanno S, Kobayashi T, Hirano S (2009) Extrapulmonary translocation of intratracheally instilled fine and ultrafine particles via direct and alveolar macrophage-associated routes. Arch Toxicol 83(5):429–437
Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W et al (2004) Translocation of inhaled ultrafine particles to the brain. Inhalation Toxicol 16(6–7):437–445
Wilker EH, Preis SR, Beiser AS, Wolf PA, Au R, Kloog I et al (2015) Long-term exposure to fine particulate matter, residential proximity to major roads and measures of brain structure. Stroke 46(5):1161–1166
Apte JS, Marshall JD, Cohen AJ, Brauer M (2015) Addressing global mortality from ambient PM2. 5. Environ Sci Technol 49(13):8057–8066
Dai J, Sun C, Yao Z, Chen W, Yu L, Long M (2016) Exposure to concentrated ambient fine particulate matter disrupts vascular endothelial cell barrier function via the IL-6/HIF‐1α signaling pathway. FEBS open bio 6(7):720–728
Hajipour S, Farbood Y, Gharib-Naseri MK, Goudarzi G, Rashno M, Maleki H et al (2020) Exposure to ambient dusty particulate matter impairs spatial memory and hippocampal LTP by increasing brain inflammation and oxidative stress in rats. Life Sci 242:117210
Brook RD, Rajagopalan S (2010) Particulate matter air pollution and atherosclerosis. Curr Atheroscler Rep 12(5):291–300
Bhouri W, Boubaker J, Skandrani I, Ghedira K, Ghedira LC (2012) Investigation of the apoptotic way induced by digallic acid in human lymphoblastoid TK6 cells. Cancer Cell Int 12(1):1–8
Bastianetto S, Yao ZX, Papadopoulos V, Quirion R (2006) Neuroprotective effects of green and black teas and their catechin gallate esters against β-amyloid‐induced toxicity. Eur J Neurosci 23(1):55–64
Sarkaki A, Farbood Y, Mansouri SMT, Badavi M, Khorsandi L, Dehcheshmeh MG et al (2019) Chrysin prevents cognitive and hippocampal long-term potentiation deficits and inflammation in rat with cerebral hypoperfusion and reperfusion injury. Life Sci 226:202–209
Pulsinelli WA, Brierley JB (1979) A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke 10(3):267–272
Hoffmann C, Funk R, Sommer M, Li Y (2008) Temporal variations in PM10 and particle size distribution during asian dust storms in Inner Mongolia. Atmos Environ 42(36):8422–8431
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11(1):47–60
Farbood Y, Sarkaki A, Dianat M, Khodadadi A, Haddad MK, Mashhadizadeh S (2015) Ellagic acid prevents cognitive and hippocampal long-term potentiation deficits and brain inflammation in rat with traumatic brain injury. Life Sci 124:120–127
Musah S, DeJarnett N, Hoyle GW (2012) Tumor necrosis factor-α mediates interactions between macrophages and epithelial cells underlying proinflammatory gene expression induced by particulate matter. Toxicology 299(2–3):125–132
Mard SA, Akbari G, Dianat M, Mansouri E (2019) The effect of zinc sulfate on miR-122, miR-34a, Atioxidants, biochemical and histopathological parameters following hepatic ischemia/reperfusion injury in rats. Biol Trace Elem Res 188(2):434–440
Khoshnam SE, Sarkaki A, Khorsandi L, Winlow W, Badavi M, Moghaddam HF et al (2017) Vanillic acid attenuates effects of transient bilateral common carotid occlusion and reperfusion in rats. Biomed Pharmacother 96:667–674
Fang C, Li Q, Min G, Liu M, Cui J, Sun J et al (2017) MicroRNA-181c ameliorates cognitive impairment induced by chronic cerebral hypoperfusion in rats. Mol Neurobiol 54(10):8370–8385
Xue R, Wu G, Wei X, Lv J, Fu R, Lei X et al (2016) Tea polyphenols may attenuate the neurocognitive impairment caused by global cerebral ischemia/reperfusion injury via anti-apoptosis. Nutr Neurosci 19(2):63–69
Ten VS, Wu EX, Tang H, Bradley-Moore M, Fedarau MV, Ratner VI et al (2004) Late measures of brain injury after neonatal hypoxia–ischemia in mice. Stroke 35(9):2183–2188
Farbood Y, Sarkaki A, Hashemi S, Mansouri MT, Dianat M (2013) The effects of gallic acid on pain and memory following transient global ischemia/reperfusion in Wistar rats. Avicenna J Phytomed 3(4):329
Cechetti F, Pagnussat AS, Worm PV, Elsner VR, Ben J, da Costa MS et al (2012) Chronic brain hypoperfusion causes early glial activation and neuronal death, and subsequent long-term memory impairment. Brain Res Bull 87(1):109–116
Ailshire JA, Crimmins EM (2014) Fine particulate matter air pollution and cognitive function among older US adults. Am J Epidemiol 180(4):359–366
Morgan JA, Singhal G, Corrigan F, Jaehne EJ, Jawahar MC, Baune BT (2018) TNF signalling via the TNF receptors mediates the effects of exercise on cognition-like behaviours. Behav Brain Res 353:74–82
Taylor DL, Jones F, Kubota ESCS, Pocock JM (2005) Stimulation of microglial metabotropic glutamate receptor mGlu2 triggers tumor necrosis factor α-induced neurotoxicity in concert with microglial-derived Fas ligand. J Neurosci 25(11):2952–2964
Khoshnam SE, Sarkaki A, Rashno M, Farbood Y (2018) Memory deficits and hippocampal inflammation in cerebral hypoperfusion and reperfusion in male rats: neuroprotective role of vanillic acid. Life Sci 211:126–132
Sabat R, Grütz G, Warszawska K, Kirsch S, Witte E, Wolk K et al (2010) Biology of interleukin-10. Cytokine Growth Factor Rev 21(5):331–344
Spera PA, Ellison JA, Feuerstein GZ, Barone FC (1998) IL-10 reduces rat brain injury following focal stroke. Neurosci Lett 251(3):189–192
Wang X, Zhang X, Ren X-P, Chen J, Liu H, Yang J et al (2010) MicroRNA-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardiac injury. Circulation 122(13):1308–1318
Peng B, Guo Q-l, He Z-j, Ye Z, Yuan Y-j, Wang N et al (2012) Remote ischemic postconditioning protects the brain from global cerebral ischemia/reperfusion injury by up-regulating endothelial nitric oxide synthase through the PI3K/Akt pathway. Brain Res 1445:92–102
Ma Q, Zhao H, Tao Z, Wang R, Liu P, Han Z et al (2016) MicroRNA-181c exacerbates brain injury in acute ischemic stroke. Aging and disease 7(6):705
Miao W, Yan Y, Bao T-h, Jia W-j, Yang F, Wang Y et al (2020) Ischemic postconditioning exerts neuroprotective effect through negatively regulating PI3K/Akt2 signaling pathway by microRNA-124. Biomed Pharmacother 126:109786
He X, Zheng Y, Liu S, Shi S, Liu Y, He Y et al (2018) MiR-146a protects small intestine against ischemia/reperfusion injury by down‐regulating TLR4/TRAF6/NF‐κB pathway. J Cell Physiol 233(3):2476–2488
Nguyen LS, Fregeac J, Bole-Feysot C, Cagnard N, Iyer A, Anink J et al (2018) Role of miR-146a in neural stem cell differentiation and neural lineage determination: relevance for neurodevelopmental disorders. Mol autism 9(1):1–12
Qu X, Wang N, Cheng W, Xue Y, Chen W, Qi M (2019) MicroRNA–146a protects against intracerebral hemorrhage by inhibiting inflammation and oxidative stress. Exp Ther Med 18(5):3920–3928
Acknowledgements
We are grateful to Dr. Mohammad Rashno for his contribution and scientific support.
Funding
This article was extracted from Kowsar Bavarsad’s post-Doc thesis (PD-APRC-9701). Research reported in this publication was supported by the Elite Researchers Grant Committee under award number [Grant No. 971250] from the National Institute for Medical Research Development (NIMAD), Tehran, Iran. This work was carried out in the Persian Gulf Physiology Research Center and was partially funded by Research Affaires Deputy of Ahvaz Jundishapur University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
KB: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Writing—Original Draft, Writing—Review & Editing, Visualization. YF: Conceptualization, Methodology, Visualization. SEK: Conceptualization, Methodology, Visualization. SAM: Methodology, Formal analysis, Investigation, Writing—Review & Editing, Visualization. MD: Conceptualization, Investigation, Resources, Visualization. HMJ: Conceptualization, Methodology, Formal analysis, Investigation. LSK: Methodology, Formal analysis, Investigation, Resources. GG: Methodology, Formal analysis, Investigation, Visualization. AS: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Writing—Review & Editing, Visualization, Supervision, Project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bavarsad, K., Farbood, Y., Mard, S.A. et al. Effects of Gallic Acid on Memory Deficits and Electrophysiological Impairments Induced by Cerebral Ischemia/Reperfusion in Rats Following Exposure to Ambient Dust Storm. Neurochem Res 48, 2911–2923 (2023). https://doi.org/10.1007/s11064-023-03953-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-03953-5