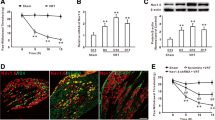
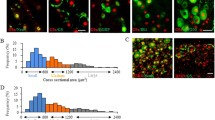
Abstract
Sympathetic axonal sprouting into dorsal root ganglia is a major phenomenon implicated in neuropathic pain, and sympathetic ganglia blockage may relieve some intractable chronic pain in animal pain models and clinical conditions. These suggest that sympathetic ganglia participated in the maintenance of chronic pain. However, the molecular mechanism underlying sympathetic ganglia-mediated chronic pain is not clear. Here, we found that spared nerve injury treatment upregulated the expression of ADAMTS4 and AP-2α protein and mRNA in the noradrenergic neurons of sympathetic ganglia during neuropathic pain maintenance. Knockdown the ADAMTS4 or AP-2α by injecting specific retro scAAV-TH (Tyrosine Hydroxylase)-shRNA ameliorated the mechanical allodynia induced by spared nerve injury on day 21 and 28. Furthermore, chromatin immunoprecipitation and coimmunoprecipitation assays found that spared nerve injury increased the recruitment of AP-2α to the ADAMTS4 gene promoter, the interaction between AP-2α and histone acetyltransferase p300 and the histone H4 acetylation on day 28. Finally, knockdown the AP-2α reduced the acetylation of H4 on the promoter region of ADAMTS4 gene and suppressed the increase of ADAMTS4 expression induced by spared nerve injury. Together, these results suggested that the enhanced interaction between AP-2α and p300 mediated the epigenetic upregulation of ADAMTS4 in sympathetic ganglia noradrenergic neurons, which contributed to the maintenance of spared nerve injury induced neuropathic pain.




Similar content being viewed by others
Data Availability
The datasets analyzed during the current study are not publicly available since another ongoing study still using certain data but are available from the corresponding author on reasonable request.
References
Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, Gao YJ, Roy K, Corfas G, Lo EH, Ji RR (2008) Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med 14:331–336. https://doi.org/10.1038/nm1723
Finnerup NB, Kuner R, Jensen TS (2021) Neuropathic Pain: from mechanisms to treatment. Physiol Rev 101:259–301. https://doi.org/10.1152/physrev.00045.2019
Ji RR, Strichartz G (2004) Cell signaling and the genesis of neuropathic pain. Sci STKE. https://doi.org/10.1126/stke.2522004re14
Cohen SP, Vase L, Hooten WM (2021) Chronic pain: an update on burden, best practices, and new advances. Lancet (London England) 397:2082–2097. https://doi.org/10.1016/S0140-6736(21)00393-7
Torrance N, Smith BH, Bennett MI, Lee AJ (2006) The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain 7:281–289. https://doi.org/10.1016/j.jpain.2005.11.008
O’Connor AB, Dworkin RH (2009) Treatment of neuropathic pain: an overview of recent guidelines. Am J Med 122:S22–32. https://doi.org/10.1016/j.amjmed.2009.04.007
Chien SQ, Li C, Li H, Xie W, Pablo CS, Zhang JM (2005) Sympathetic Fiber sprouting in chronically compressed dorsal Root Ganglia without Peripheral Axotomy. J Neuropathic Pain Symptom Palliation 1:19–23. https://doi.org/10.1300/J426v01n01_05
Xie W, Strong JA, Zhang JM (2020) Localized sympathectomy reduces peripheral nerve regeneration and pain behaviors in 2 rat neuropathic pain models. Pain 161:1925–1936. https://doi.org/10.1097/j.pain.0000000000001887
Harden RN, McCabe CS, Goebel A, Massey M, Suvar T, Grieve S, Bruehl S (2022) Complex regional pain syndrome: Pain Med (Malden, Mass) 23:S1–S53. https://doi.org/10.1093/pm/pnac046
Drummond PD (2013) Sensory-autonomic interactions in health and disease. Handb Clin Neurol 117:309–319. https://doi.org/10.1016/B978-0-444-53491-0.00024-9
Xie W, Chen S, Strong JA, Li AL, Lewkowich IP, Zhang JM (2016) Localized Sympathectomy reduces mechanical hypersensitivity by restoring normal Immune Homeostasis in Rat Models of Inflammatory Pain. J Neurosci 36:8712–8725. https://doi.org/10.1523/JNEUROSCI.4118-15.2016
Li H, Shang M, Liu L, Lin X, Hu J, Han Q, Xing J (2021) Protein kinase G signaling pathway is involved in sympathetically maintained pain by modulating ATP-sensitive potassium channels. Reg Anesth Pain Med 46:1006–1011. https://doi.org/10.1136/rapm-2021-102539
Mantyh PW (2014) The neurobiology of skeletal pain. Eur J Neurosci 39:508–519. https://doi.org/10.1111/ejn.12462
Schlereth T, Drummond PD, Birklein F (2014) Inflammation in CRPS: role of the sympathetic supply. Auton Neurosci 182:102–107. https://doi.org/10.1016/j.autneu.2013.12.011
Rose KWJ, Taye N, Karoulias SZ, Hubmacher D (2021) Regulation of ADAMTS Proteases. Front Mol Biosci 8:701959. https://doi.org/10.3389/fmolb.2021.701959
Rosenzweig DH, Quinn TM, Haglund L (2014) Low-frequency high-magnitude mechanical strain of articular chondrocytes activates p38 MAPK and induces phenotypic changes associated with osteoarthritis and pain. Int J Mol Sci 15:14427–14441. https://doi.org/10.3390/ijms150814427
Shu CC, Dart A, Bell R, Dart C, Clarke E, Smith MM, Little CB, Melrose J (2018) Efficacy of administered mesenchymal stem cells in the initiation and co-ordination of repair processes by resident disc cells in an ovine (Ovis aries) large destabilizing lesion model of experimental disc degeneration. JOR Spine 1:e1037. https://doi.org/10.1002/jsp2.1037
Klein K, Habiger C, Iftner T, Stubenrauch F (2020) A TGF-beta- and p63-Responsive enhancer regulates IFN-kappa expression in human keratinocytes. J Immunol 204:1825–1835. https://doi.org/10.4049/jimmunol.1901178
Li H, Li W, Liang B, Wei J, Yin D, Fan Q (2020) Role of AP-2alpha/TGF-beta1/Smad3 axis in rats with intervertebral disc degeneration. Life Sci 263:118567. https://doi.org/10.1016/j.lfs.2020.118567
Shvedunova M, Akhtar A (2022) Modulation of cellular processes by histone and non-histone protein acetylation. Nat Rev Mol Cell Biol 23:329–349. https://doi.org/10.1038/s41580-021-00441-y
Decosterd I, Woolf CJ (2000) Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87:149–158. https://doi.org/10.1016/S0304-3959(00)00276-1
Kolat D, Kaluzinska Z, Bednarek AK, Pluciennik E (2019) The biological characteristics of transcription factors AP-2alpha and AP-2gamma and their importance in various types of cancers. Biosci Rep. https://doi.org/10.1042/BSR20181928
Li N, Rivera-Bermudez MA, Zhang M, Tejada J, Glasson SS, Collins-Racie LA, Lavallie ER, Wang Y, Chang KC, Nagpal S, Morris EA, Flannery CR, Yang Z (2010) LXR modulation blocks prostaglandin E2 production and matrix degradation in cartilage and alleviates pain in a rat osteoarthritis model. Proc Natl Acad Sci U S A 107:3734–3739. https://doi.org/10.1073/pnas.0911377107
Lemarchant S, Wojciechowski S, Vivien D, Koistinaho J (2017) ADAMTS-4 in central nervous system pathologies. J Neurosci Res 95:1703–1711. https://doi.org/10.1002/jnr.24021
Lemarchant S, Pruvost M, Hebert M, Gauberti M, Hommet Y, Briens A, Maubert E, Gueye Y, Feron F, Petite D, Mersel M, do Rego JC, Vaudry H, Koistinaho J, Ali C, Agin V, Emery E, Vivien D (2014) tPA promotes ADAMTS-4-induced CSPG degradation, thereby enhancing neuroplasticity following spinal cord injury. Neurobiol Dis 66:28–42. https://doi.org/10.1016/j.nbd.2014.02.005
Tauchi R, Imagama S, Natori T, Ohgomori T, Muramoto A, Shinjo R, Matsuyama Y, Ishiguro N, Kadomatsu K (2012) The endogenous proteoglycan-degrading enzyme ADAMTS-4 promotes functional recovery after spinal cord injury. J Neuroinflammation 9:53. https://doi.org/10.1186/1742-2094-9-53
Miller RE, Tran PB, Ishihara S, Larkin J, Malfait AM (2016) Therapeutic effects of an anti-ADAMTS-5 antibody on joint damage and mechanical allodynia in a murine model of osteoarthritis. Osteoarthritis Cartilage 24:299–306. https://doi.org/10.1016/j.joca.2015.09.005
Zhao P, Liu D, Song CY, Li D, Zhang XZ, Horecny I, Zhang FQ, Yan YN, Zhuang LH, Li J, Liu SX, Mao YC, Feng J, Liu J, Tao WK (2022) Discovery of Isoindoline Amide derivatives as potent and orally bioavailable ADAMTS-4/5 inhibitors for the treatment of Osteoarthritis. Acs Pharmacol Transl. https://doi.org/10.1021/acsptsci.2c00023
Lemarchant S, Dunghana H, Pomeshchik Y, Leinonen H, Kolosowska N, Korhonen P, Kanninen KM, Garcia-Berrocoso T, Montaner J, Malm T, Koistinaho J (2016) Anti-inflammatory effects of ADAMTS-4 in a mouse model of ischemic stroke. Glia 64:1492–1507. https://doi.org/10.1002/glia.23017
Hilger-Eversheim K, Moser M, Schorle H, Buettner R (2000) Regulatory roles of AP-2 transcription factors in vertebrate development, apoptosis and cell-cycle control. Gene 260:1–12. https://doi.org/10.1016/s0378-1119(00)00454-6
Descalzi G, Mitsi V, Purushothaman I, Gaspari S, Avrampou K, Loh YE, Shen L, Zachariou V (2017) Neuropathic pain promotes adaptive changes in gene expression in brain networks involved in stress and depression. Sci Signal. https://doi.org/10.1126/scisignal.aaj1549
Santoso CS, Li Z, Lal S, Yuan S, Gan KA, Agosto LM, Liu X, Pro SC, Sewell JA, Henderson A, Atianand MK, Fuxman Bass JI (2020) Comprehensive mapping of the human cytokine gene regulatory network. Nucleic Acids Res 48:12055–12073. https://doi.org/10.1093/nar/gkaa1055
Carrasco Pro S, Dafonte Imedio A, Santoso CS, Gan KA, Sewell JA, Martinez M, Sereda R, Mehta S, Fuxman Bass JI (2018) Global landscape of mouse and human cytokine transcriptional regulation. Nucleic Acids Res 46:9321–9337. https://doi.org/10.1093/nar/gky787
Rothstein M, Simoes-Costa M (2020) Heterodimerization of TFAP2 pioneer factors drives epigenomic remodeling during neural crest specification. Genome Res 30:35–48. https://doi.org/10.1101/gr.249680.119
Acknowledgements
This study was funded by National Natural Science Foundation of China (Grant No. 31970936, 81870829, 82071224, 62175263), Natural Science Foundation of Guangdong (2019A1515010871, 2019A1515011447, 2022A1515012259), Guangzhou Science and Technology Plan Project (202206060004, 202002020011), Major Project of Basic and Applied Basic Research Foundation of Guangdong Province (2019B1515120054).
Funding
This study was funded by National Natural Science Foundation of China (Grant No.31970936, 81870829, 82071224, 62175263), Natural Science Foundation of Guangdong (2019A1515010871, 2019A1515011447, 2022A1515012259), Guangzhou Science and Technology Plan Project (202206060004, 202002020011), Major Project of Basic and Applied Basic Research Foundation of Guangdong Province (2019B1515120054).
Author information
Authors and Affiliations
Contributions
XF and W-JX contributed to the study conception and design. Data collection and analysis were performed by CW and MW. The first draft of the manuscript was written by CW and MW. Material preparation were performed by YW. Experiments were performed by H-TF, Zheng-Kai Liang and A-RL. all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interests.
Ethical Approval
The experimental protocols were approved by the Animal Care and Use Committee of Sun Yat-sen University and conducted in strict accordance with the guideline of National Institutes of Health on the animal care and the ethical guideline.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, C., Wei, M., Wu, Y. et al. Epigenetic Up-Regulation of ADAMTS4 in Sympathetic Ganglia is Involved in the Maintenance of Neuropathic Pain Following Nerve Injury. Neurochem Res 48, 2350–2359 (2023). https://doi.org/10.1007/s11064-023-03896-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-03896-x