Abstract
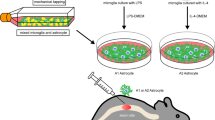
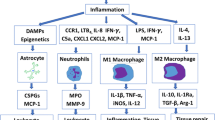
Spinal cord injury (SCI) is a serious injury to the central nervous system that causes significant physical and psychological trauma to the patient. SCI includes primary spinal cord injuries and secondary spinal cord injuries. The secondary injury refers to the pathological process or reaction after the primary injury. Although SCI has always been thought to be an incurable injury, the human nerve has the ability to repair itself after an injury. However, the reparability is limited because glial scar formation impedes functional recovery. There is a type of astrocyte that can differentiate into two forms of reactive astrocytes known as ‘A1’ and ‘A2’ astrocytes. A1 astrocytes release cytotoxic chemicals that cause neurons and oligodendrocytes to die and perform a harmful role. A2 astrocytes can produce neurotrophic factors and act as neuroprotectors. This article discusses ways to block A1 astrocytes while stimulating A2 astrocytes to formulate a new treatment for spinal cord injury.







Similar content being viewed by others
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
Perrouin-Verbe, Physical BJAo, Medicine R (2012) Epidemiology of traumatic spinal cord injury: new trends. Spinal cord 55:e165
Letton RW (2011) Spine trauma. Fundamentals of pediatric surgery. Springer
Devivo MJ (2012) Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord 50:365–372
Okada S, Maeda T, Ohkawa Y, Harimaya K, Saiwai H, Kumamaru H, Matsumoto Y, Doi T, Ueta T, Shiba KJS (2009) Does ossification of the posterior longitudinal ligament affect the neurological outcome after traumatic cervical cord injury? Spine 34:1148–1152
Anjum A, Yazid M, Daud MF, Idris J, Lokanathan YJIJoMS, (2020) Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci 21:7533
Eli I, Lerner DP, Ghogawala ZJNC (2021) Acute traumatic spinal cord injury. Neurol Clin 39:471
Hellenbrand DJ, Quinn CM, Piper ZJ, Morehouse CN, Fixel JA, Hanna AS (2021) Inflammation after spinal cord injury: a review of the critical timeline of signaling cues and cellular infiltration. J Neuroinflamm 18:284
Baroncini A, Maffulli N, Eschweiler J, Tingart M, Migliorini F (2021) Pharmacological management of secondary spinal cord injury. Expert Opin Pharmacother 22:1793–1800
Firat T, Kukner A, Ayturk N, Gezici AR, Serin E, Ozogul C, Tore F (2021) The Potential Therapeutic Effects of Agmatine, Methylprednisolone, and Rapamycin on Experimental Spinal Cord Injury. Cell J 23:701–707
Ylmaz T, Kaptanolu E (2015) Current and future medical therapeutic strategies for the functional repair of spinal cord injury. World J Orthop 6:42–55
Huang L, Fu C, Xiong F, He C, Wei Q (2021) Stem cell therapy for spinal cord Injury. Cell Transplant 30:963689721989266
Feng Y, Li Y, Shen PP, Wang B (2022) Gene-modified stem cells for spinal cord injury: a promising better alternative therapy. Stem cell Rev Rep 18:2662
Fan B, Wei Z, Xue Y, Shi G, Feng SJCT (2018) Microenvironment imbalance of spinal cord injury. Cell Transplant 27:963689718755778
Silver J, Miller JH (2004) Regeneration beyond the glial scar. Nat Rev Neuro 5:146–156
Orr MB, Gensel JC (2018) Spinal cord injury scarring and inflammation: therapies targeting glial and inflammatory responses. Neurotherapeutics 15:541–553
Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, Smith SJ, Barres BA (2013) Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504:394–400
Sofroniew MV, Vinters HVJAN (2010) Astrocytes: biology and pathology. Acta Neuropathol 119:7
Neuron BBJ (2008) The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60:430–440
Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA (2012) Neurotoxic reactive astrocytes are induced by activated microglia. Genomic Anal React Astrogliosis 32:6391–6410
Sofroniew MVJCSHPiB, (2015) Astrogliosis. Cold Spring Harb Perspect Biol 7:a020420
Okada S, Hara M, Kobayakawa K, Matsumoto Y, Nakashima Y (2017) Astrocyte reactivity and astrogliosis after spinal cord injury. Neurosci Res 126:39
Liddelow SA, Barres BA (2017) Reactive astrocytes: production, function, and therapeutic potential. Immunity 46:957–967
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch A, Chung WS, Peterson TC (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481
Yu D, Cheng Z, Ali AI, Wang J, Le K, Chibaatar E, Guo Y (2019) Down-expressed GLT-1 in PSD astrocytes inhibits synaptic formation of NSC-derived neurons in vitro. Cell Cycle (Georgetown Tex) 18:105–114
Ponath G, Ramanan S, Mubarak M, Housley W, Lee S, Sahinkaya FR, Vortmeyer A, Raine CS, Pitt D (2017) Myelin phagocytosis by astrocytes after myelin damage promotes lesion pathology. Brain 140:399–413
Okada S, Hara M, Kobayakawa K, Matsumoto Y, Nakashima Y (2018) Astrocyte reactivity and astrogliosis after spinal cord injury. Neurosci Res 126:39–43
Li L, Li Y, He B, Li H, Ji H, Wang Y, Zhu Z, Hu Y, Zhou Y, Yang T, Sun C, Yuan Y, Wang Y (2021) HSF1 is involved in suppressing A1 phenotype conversion of astrocytes following spinal cord injury in rats. J Neuroinflamm 18:205
Wang X, Zhang Z, Zhu Z, Liang Z, Zuo X, Ju C, Song Z, Li X, Hu X, Wang Z (2021) Photobiomodulation promotes repair following spinal cord injury by regulating the transformation of A1/A2 reactive astrocytes. Front Neurosci 15:768262
Banchereau J, Pascual V, O’Garra A (2012) From IL-2 to IL-37: the expanding spectrum of anti-inflammatory cytokines. Nat Immunol 13:925
Zhang HY, Wang Y, He Y, Wang T, Jiang XX (2020) A1 astrocytes contribute to murine depression-like behavior and cognitive dysfunction, which can be alleviated by IL-10 or fluorocitrate treatment. J Neuroinflammation 17:200
Yang J, Jiang Z, Fitzgerald DC, Ma C, Yu S, Li H, Zhao Z, Li Y, Ciric B, Curtis M (2009) Adult neural stem cells expressing IL-10 confer potent immunomodulation and remyelination in experimental autoimmune encephalitis. J Clin Invest 119:3678–3691
Guillot-Sestier MV, Doty K, Gate D, Rodriguez J, Leung B, Rezai-Zadeh K, Town T (2015) Il10 deficiency rebalances innate immunity to mitigate Alzheimer-like pathology. Neuron 85:534
Kanneganti TD, Body-Malapel M, Amer A, Park JH (2007) Critical role for cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem 281:36560–36568
Chen ML, Cao H, Chu YX, Cheng LZ, Liang LL, Zhang YQ, Zhao ZQ (2012) Role of P2X7 receptor-mediated IL-18/IL-18R signaling in morphine tolerance: multiple glial-neuronal dialogues in the rat spinal cord. J Pain 13:945–958
Zhang C, Kenian S, Bennett SA, Mariko L, Scholze AR, O’Keeffe S (2014) An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34:11929
Hou B, Zhang Y, Liang P, He Y, He X (2020) Inhibition of the NLRP3-inflammasome prevents cognitive deficits in experimental autoimmune encephalomyelitis mice via the alteration of astrocyte phenotype. Cell Death Dis 11:377
Alexianu ME (2001) Immune reactivity in a mouse model of familial ALS correlates with disease progression. Neurology 57:1282–1289
Lambertsen KL, Meldgaard M, Ladeby R, Finsen B (2005) A quantitative study of microglial-macrophage synthesis of tumor necrosis factor during acute and late focal cerebral ischemia in mice. J Cereb Blood Flow Metab 25:119–135
You L, Yang C, Du Y, Wang W, Ni J (2020) A systematic review of the pharmacology, toxicology and pharmacokinetics of matrine. Front Pharmacol 11:01067
Rashid HU, Xu Y, Muhammad Y, Wang L, Jiang J (2019) Research advances on anticancer activities of matrine and its derivatives: an updated overview. Eur J Med Chem 161:205
Yilei J, Rui M, Chu Y, Mda B, Dou M, Wang M, Li X (2021) Matrine treatment induced an A2 astrocyte phenotype and protected the blood-brain barrier in CNS autoimmunity. J Chem Neuroanat 117
Shahidi F (1996) Technology Bailey’s industrial oil and fat products. In: Hui YH (ed) Edible oil and fat products: general applications. John Wiley & Sons
Park JS, Choi JW, Hwang SH, Kim JK, Cho ML (2019) Cottonseed oil protects against intestinal inflammation in dextran sodium sulfate-induced inflammatory bowel disease. J Med Food 22:672
Wilhelmi G (1993) Potential effects of nutrition including additives on healthy and arthrotic joints. I. Basic dietary constituents. Z Rheumatol 52:174–179
Liu M, Xu Z, Wang L, Zhang L, Ma Y (2020) Cottonseed oil alleviates ischemic stroke injury by inhibiting the inflammatory activation of microglia and astrocyte. J Neuroinflammation 17:270
Mahban R, Faheem M, Shermineh MN, Kamal N (2017) Targeting the TLR4 signaling pathway by polyphenols: a novel therapeutic strategy for neuroinflammation. Ageing Res Rev 36:11
Ridder DA, Schwaninger M (2009) NF-κB signaling in cerebral ischemia. Neuroscience 158:995
Paudel YN, Shaikh MF, Chakraborti A, Kumari Y, Aledo-Serrano Á, Aleksovska K, Alvim MKM, Othman I (2018) HMGB1: a common biomarker and potential target for TBI, neuroinflammation, epilepsy, and cognitive dysfunction. Front NeuroSci 12:628
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418:191–195
Okuma Y, Date I, Liu K, Wake H, Haruma J, Yoshino T, Ohtsuka A, Takahashi HK, Mori S, Nishibori M (2012) Anti-high mobility group box-1 antibody therapy for traumatic brain injury. Ann Neurol 72:373–384
Kim SW, Lee H, Lee HK, Kim ID, Lee JK (2019) Neutrophil extracellular trap induced by HMGB1 exacerbates damages in the ischemic brain. Acta Neuropathol Commun 7:94
Walker LE, Frigerio F, Ravizza T, Ricci E (2017) Molecular isoforms of high-mobility group box 1 are mechanistic biomarkers for epilepsy. J Clin Invest 127:2118
Kigerl KA, Lai W, Wallace LM, Yang H, Popovich PG (2017) High mobility group box-1 (HMGB1) is increased in injured mouse spinal cord and can elicit neurotoxic inflammation. Brain Behav Immun 72:22
Andersson U, Yang H, Harris H (2018) High-mobility group box 1 protein (HMGB1) operates as an alarmin outside as well as inside cells. Semin Immunol 38:40
Hong F (2020) Inhibiting HMGB1-RAGE axis prevents pro-inflammatory macrophages/microglia polarization and affords neuroprotection after spinal cord injury. J Neuroinflamm 17:295
Musumeci D, Roviello GN, Montesarchio D (2014) An overview on HMGB1 inhibitors as potential therapeutic agents in HMGB1-related pathologies. Pharmacol Ther 141:347–357
Gong G, Yuan LB, Ling HU, Wei WL, Yin L, Hou JL, Liu YH, Zhou LS (2012) Glycyrrhizin attenuates rat ischemic spinal cord injury by suppressing inflammatory cytokines and HMGB1. Acta Pharmacol Sin 33:8
Gabay C, Towne JE (2015) Regulation and function of interleukin-36 cytokines in homeostasis and pathological conditions. J Leukoc Biol 97:645
Dawn Q, Ediriweera C, Liang L (2019) Function and regulation of IL-36 signaling in inflammatory diseases and cancer development. Front Cell Dev Biol 7:317–317
Xia X, Jiang Q, Mcdermott J, Han JD (2018) Aging and Alzheimer’s disease: comparison and associations from molecular to system level. Aging Cell 17
Kant R, Goldstein LSB (2015) Cellular functions of the amyloid precursor protein from development to dementia. Dev Cell 32:502
Reinhard C, Habert SS, Strooper BD (2005) The amyloid-β precursor protein: integrating structure with biological function. EMBO J 24:3996–4006
Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT (2018) Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dementia (N Y) 4:575–590
Habib N, Mccabe C, Medina S, Varshavsky M, Schwartz M (2020) Disease-associated astrocytes in Alzheimer’s disease and aging. Nat Neurosci 23:1–6
Hemonnot AL, Hua J, Ulmann L, Hirbec H (2019) Microglia in Alzheimer disease: well-known targets and new opportunities. Front Aging Neurosci 11:233
Larocca T, Cavalier AN, Roberts CM, Lemieux MR, Link CD (2021) Amyloid beta acts synergistically as a pro-inflammatory cytokine. Neurobiol Dis 159:105493
Chen Y, Durakoglugil MS, Xian X, Herz J (2010) ApoE4 reduces glutamate receptor function and synaptic plasticity by selectively impairing ApoE receptor recycling. Proc Natl Acad Sci U S A 107:12011
Dibattista AM, Heinsinger NM, Rebeck GW (2016) Alzheimer’s disease genetic risk factor APOE-ε4 also affects normal brain function. Curr Alzheimer Res 13:1200
Holtzman DM, Herz J, Bu GJ (2012) Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med 2
Castellano JM, Deane R, Gottesdiener AJ, Verghese PB, Stewart FR, West T, Paoletti AC, Kasper TR, Demattos RB, Zlokovic BV (2012) Low-density lipoprotein receptor overexpression enhances the rate of brain-to-blood Aβ clearance in a mouse model of β-amyloidosis. Proc Natl Acad Sci U S A 109:15502
Fryer JD, Demattos RB, Mccormick LM, O’dell MA, Spinner ML, Bales KR, Paul SM, Sullivan PM, Parsadanian M, Bu G (2005) The low density lipoprotein receptor regulates the level of central nervous system human and murine apolipoprotein E but does not modify amyloid plaque pathology in PDAPP mice. J Biol Chem 280:25754–25759
Kim J, Castellano JM, Jiang H, Basak JM, Parsadanian M, Pham V, Mason SM, Paul SM, Holtzman DM (2009) Overexpression of low-density lipoprotein receptor in the brain markedly inhibits amyloid deposition and increases extracellular A beta clearance. Neuron 64:632–644
Kuszczyk MA, Sanchez S, Pankiewicz J, Kim J, Duszczyk M, Guridi M, Asuni AA, Sullivan PM, Holtzman DM, Sadowski MJ (2013) Blocking the interaction between apolipoprotein E and Aβ reduces intraneuronal accumulation of Aβ and inhibits synaptic degeneration. Am J Pathol 182:1750–1768
Zeng H, Chen P, Chang Q, Zheng B, Zhang Y (2019) Hypolipidemic effect of polysaccharides from Fortunella margarita (Lour.) swingle in hyperlipidemic rats. Food Chem Toxicol 132:110663
Zhang Y, Pan X, Ran S, Wang K (2019) Purification, structural elucidation and anti-inflammatory activity in vitro of polysaccharides from Smilax china L. Int J Biol Macromol 139:233–243
Liu QY, Yao YM, Zhang SW, Sheng ZY (2011) Astragalus polysaccharides regulate T cell-mediated immunity via CD11c(high)CD45RB(low) DCs in vitro. J Ethnopharmacol 136:457–464
Prinz M, Jung S, Priller J (2019) Microglia biology: one century of evolving concepts. Science 179:292–311
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308:1314–1318
Gordon S (2003) Alternative activation of macrophages. Nat Rev Immunol 3:23–35
Benedek G, Zhang J, Bodhankar S, Nguyen H, Kent G, Jordan K, Manning D, Vandenbark AA, Offner H (2016) Estrogen induces multiple regulatory B cell subtypes and promotes M2 microglia and neuroprotection during experimental autoimmune encephalomyelitis. J Neuroimmunol 293:45–53
Mikita J, Dubourdieu-Cassagno N, Deloire MS, Vekris A, Biran M, Raffard G, Brochet B, Canron MH, Franconi JM, Boiziau C (2011) Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of multiple sclerosis. Amelioration of clinical status by M2 activated monocyte administration. Mult Scler 17:2–15
Dickey LL, Hanley TM, Huffaker TB, Ramstead AG, O’Connell RM, Lane TE (2017) MicroRNA 155 and viral-induced neuroinflammation. J Neuroimmunol 308:17–24
Liu X, Ma J, Ding G, Gong Q, Wang Y, Yu H, Cheng X (2021) Microglia polarization from M1 toward M2 phenotype is promoted by astragalus polysaccharides mediated through inhibition of miR-155 in experimental autoimmune encephalomyelitis. Oxid Med Cell Longev 2021:5753452
Meikuang L, Weiqiang W, Lu L, Zhi-Bo H, Zongjin L, Jie G, Meng Z, Honghong J, Jie F, Zhe W (2018) Intravenous injection of allogeneic umbilical cord-derived multipotent mesenchymal stromal cells reduces the infarct area and ameliorates cardiac function in a porcine model of acute myocardial infarction. Stem Cell Res Ther 9:129
Rostami S, Zabihi E, Ghasemi-Kasman M (2019) The potential use of mesenchymal stem cells for the treatment of multiple sclerosis. Life Sci 235
Squillaro T, Peluso G, Galderisi U (2016) Clinical trials with mesenchymal stem cells: an update. Cell Transplant 25:829–848
Tahmasebi F, Pasbakhsh P, Barati S, Madadi S, Kashani IR (2020) The effect of microglial ablation and mesenchymal stem cell transplantation on a cuprizone-induced demyelination model. J Cell Physiol 236:3552
Barati S, Kashani IR, Tahmasebi F (2022) The effects of mesenchymal stem cells transplantation on A1 neurotoxic reactive astrocyte and demyelination in the cuprizone model. J Mol Histol 53:333
Zhang X, He Q, Huang T, Zhao N, Liang F, Xu B, Chen X, Li T (2019) Treadmill exercise decreases Aβ deposition and counteracts cognitive decline in APP/PS1 mice, possibly via hippocampal microglia modifications. Front Aging Neurosci 11:78
Smith MA, Rottkamp CA, Nunomura A, Raina AK, Perry G (2000) Oxidative stress in Alzheimer’s disease. Biochim Biophys Acta 1502:139–144
Liu P, Fleete MS, Jing Y, Collie ND, Zhang H (2014) Altered arginine metabolism in Alzheimer’s disease brains. Neurobiol Aging 35:1992–2003
Grimaldi A, Pediconi N, Oieni F, Pizzarelli R, Angelantonio SD (2019) Neuroinflammatory processes, A1 astrocyte activation and protein aggregation in the retina of Alzheimer’s disease patients, possible biomarkers for early diagnosis. Front Neurosci 13:925
Kn A, Hs A, Kn A, So B, St B, At A, Kk CJEN (2021) Effect of low-intensity motor balance and coordination exercise on cognitive functions, hippocampal Aβ deposition, neuronal loss, neuroinflammation, and oxidative stress in a mouse model of Alzheimer’s disease. Exp Neurol 337
Packer N, Hoffman-Goetz L (2015) Acute exercise increases hippocampal TNF-α, Caspase-3 and Caspase-7 expression in healthy young and older mice. J Sports Med Phys Fitness 55:368–376
Neves J et al (2016) Immune modulation by MANF promotes tissue repair and regenerative success in the retina. J Sci. https://doi.org/10.1126/science.aaf3646
Neves J, Zhu J, Sousa-Victor P, Konjikusic M, Riley R, Chew S, Qi Y, Jasper H, Lamba DA (2016) Immune modulation by MANF promotes tissue repair and regenerative success in the retina. Science 335:3646
Mtlik K, Anttila JE, Kuan-Yin T, Smolander OP, Pakarinen E, Lehtonen L, Abo-Ramadan U, Lindholm P, Zheng C, Harvey B (2018) Poststroke delivery of MANF promotes functional recovery in rats. Sci Adv. https://doi.org/10.1126/sciadv.aap8957
Glembotski CC, Thuerauf DJ, Huang C, Vekich JA, Gottlieb RA, Doroudgar S (2012) Mesencephalic astrocyte-derived neurotrophic factor protects the heart from ischemic damage and is selectively secreted upon sarco/endoplasmic reticulum calcium depletion. J Biol Chem 287:25893–25904
Lu J, Luo L, Huang D, Xian L, Li Y (2018) Photoreceptor protection by mesencephalic astrocyte-derived neurotrophic factor (MANF). eNeuro. https://doi.org/10.1523/ENEURO.0109-18.2018
Ding Y, Liu B, Yang Z, Fang F, Li X, Song WB (2021) Hydrogen sulphide protects mice against the mutual aggravation of cerebral ischaemia/reperfusion injury and colitis. Eur J Pharmacol. https://doi.org/10.1016/j.ejphar.2021.174682
Han B, Zhang Y, Zhang Y, Bai Y, Chen X, Huang R, Wu F, Leng S, Chao J, Zhang JHJA (2018) Novel insight into circular RNA HECTD1 in astrocyte activation via autophagy by targeting MIR142-TIPARP: implications for cerebral ischemic stroke. Autophagy 14:1164
Feng Z, Zhang L, Wang S, Hong Q (2020) Circular RNA circDLGAP4 exerts neuroprotective effects via modulating miR-134-5p/CREB pathway in Parkinson’s disease. Science 522:388–394
Lu Y, Tan L, Wang X (2019) Circular HDAC9/microRNA-138/Sirtuin-1 pathway mediates synaptic and amyloid precursor protein processing deficits in Alzheimer’s disease. Neurosci Bull 35:887
Dube U, Del-Aguila JL, Li Z, Budde JP, Cruchaga C (2019) An atlas of cortical circular RNA expression in Alzheimer disease brains demonstrates clinical and pathological associations. Nat Neurosci 22:1903
Shao L, Jiang GT, Yang XL, Zeng ML, Chen JJ, Kong S et al (2021) Silencing of circIgf1r plays a protective role in neuronal injury via regulating astrocyte polarization during epilepsy. FASEB J 35:1–10
Storm JF (1987) Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol 385:733
Jin MM, Wang F, Qi D, Liu WW, Liu CF (2018) A critical role of autophagy in regulating microglia polarization in neurodegeneration. Front Aging Neurosci 10:378
Ray SK (2020) Modulation of autophagy for neuroprotection and functional recovery in traumatic spinal cord injury. Neural Regen Res 15:12
Gao M, Xiao L, Chen W, Xiao G, Zhang Y, Yu R, Li J (2018) Randomized clinical trial of physiological ischemic training for patients with coronary heart disease complicated with heart failure: safety of training, VEGF of peripheral blood and quality of life. Exp Ther Med 16:260
Liao Z, Bu Y, Li M, Han R, Zhang N, Hao J, Jiang WJBN (2019) Remote ischemic conditioning improves cognition in patients with subcortical ischemic vascular dementia. BMC Neurol 19:206
Wang Y, Zhang Z, Zhang L, Yang H, Shen Z (2018) RLIPostC protects against cerebral ischemia through improved synaptogenesis in rats. Brain Inj 32:1–8
Kerendi F, Kin H, Halkos ME, Jiang R, Zatta AJ, Zhao ZQ, Guyton RA (2005) Remote postconditioning. Brief renal ischemia and reperfusion applied before coronary artery reperfusion reduces myocardial infarct size via endogenous activation of adenosine receptors. Basic Res Cardiol 100:404
Fernández-García S, Sancho-Balsells A, Longueville S, Hervé D, Giralt A (2020) Astrocytic BDNF and TrkB regulate severity and neuronal activity in mouse models of temporal lobe epilepsy. Cell Death Dis 11:411
Paola B, Brignolo E, De Grandi E, Silvio B (2016) Supplementation with Omega-3 fatty acids in psychiatric disorders: a review of literature data. J Clin Med 5:67
Gu M, Li Y, Tang H, Zhang C, Li W, Zhang Y, Li Y, Zhao Y, Song CJN (2018) Endogenous omega (n)-3 fatty acids in Fat-1 mice attenuated depression-like behavior, imbalance between microglial M1 and M2 phenotypes, and dysfunction of neurotrophins induced by lipopolysaccharide administration. Nutrients 10:1351
Pani B, Singh BB (2009) Lipid rafts/caveolae as microdomains of calcium signaling. Cell Calcium 45:625–633
Wassall SR, Stillwell W (2009) Polyunsaturated fatty acid-cholesterol interactions: domain formation in membranes. Biochim Biophys Acta 1788:24–32
Kleindienst A, Hesse F, Bullock MR, Buchfelder MJ (2007) The neurotrophic protein S100B: value as a marker of brain damage and possible therapeutic implications. Prog Brain Res 161:317–325
Gu M, Li X, Yan L, Zhang Y, Yang L, Li S, Song C (2021) Endogenous ω-3 fatty acids in Fat-1 mice attenuated depression-like behaviors, spatial memory impairment and relevant changes induced by olfactory bulbectomy. Prostaglandins Leukot Essent Fatty Acids 171: 102313
Tan J, Mckenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L (2014) The role of short-chain fatty acids in health and disease. Adv Immunol 121:91–119
Sun J, Xu J, Ling Y, Wang F, Liu J (2019) Fecal microbiota transplantation alleviated Alzheimer’s disease-like pathogenesis in APP/PS1 transgenic mice. Transl Psychiatry 9:189
Sun J, Yuan B, Wu Y, Gong Y, Guo W, Fu S, Luan Y, Wang W (2020) Sodium butyrate protects N2a cells against Aβ toxicity in vitro. Mediators Inflamm 2020:7605160
Jiang Y, Li K, Li X, Xu L, Yang Z (2021) Sodium butyrate ameliorates the impairment of synaptic plasticity by inhibiting the neuroinflammation in 5XFAD mice. Chem Biol Interact 25:341
Wang C, Zheng D, Weng F, Jin Y, He L (2021) Sodium butyrate ameliorates the cognitive impairment of Alzheimer’s disease by regulating the metabolism of astrocytes. Psychopharmacology (Berl) 239:215
Wang L, Wu L, Duan Y, Xu S, Yang Y, Yin J, Lang Y, Gao Z, Wu C, Lv Z, Shi J, Wu D, Ji X (2022) Phenotype shifting in astrocytes account for benefits of intra-arterial selective cooling infusion in hypertensive rats of ischemic stroke. Neurotherapeutics 19:386
Kogel V, Trinh S, Gasterich N, Beyer C, Seitz J (2021) Long-term glucose starvation induces inflammatory responses and phenotype switch in primary cortical rat astrocytes. J Mol Neurosci 71:2368–2382
Huiliang Z, Mengzhe Y, Xiaochuan W, Hui W, Min D, Mengqi W, Jianzhi W, Zhongshan C, Caixia P, Rong L (2021) Zinc induces reactive astrogliosis through ERK-dependent activation of Stat3 and promotes synaptic degeneration. J Neurochem 159:1016–1027
Bruno CJ, Greco TM, Ischiropoulos H (2011) Nitric oxide counteracts the hyperoxia-induced proliferation and proinflammatory responses of mouse astrocytes. Free Radic Biol Med 51:474–479
Danilov CA, Fiskum G (2008) Hyperoxia promotes astrocyte cell death after oxygen and glucose deprivation. Glia 56:801–808
Funding
This research was supported by grants from the Sichuan Science and Technology Program (2022YFS0188), Key Science and Technology Project of Luzhou Government (2021-SYF-33), Central Nervous System Drug Key Laboratory of Sichuan Province (210020-01SZ), Science Fund of Southwest Medical University (21YYJC0246).
Author information
Authors and Affiliations
Contributions
JW: Conceptualization, Data curation, Roles/Writing - original draft. CC: Formal analysis. ZL: Data curation, Software. YL: Investigation. LY and ZZ: Methodology. XS: Visualization. PJ and MZ: Writing - review & editing. ZZ: Project administration, Writing - review & editing, Validation.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Cheng, C., Liu, Z. et al. Inhibition of A1 Astrocytes and Activation of A2 Astrocytes for the Treatment of Spinal Cord Injury. Neurochem Res 48, 767–780 (2023). https://doi.org/10.1007/s11064-022-03820-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03820-9