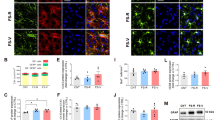
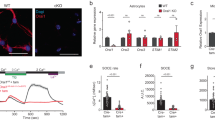
Abstract
Now astrocytes appear to be the key contributors to the pathophysiology of major depression. Evidence in rodents shows that chronic stress is associated with a decreased expression of astrocytic GFAP-immunoreactivity within the cortex in addition to changes in the complexity and length of astrocyte processes. Furthermore, postmortem brains of individuals with depression have revealed a decrease in astrocyte density. Notably, astrocytes are extensively coupled to one another through gap junctions to form a network, or syncytium, and we have previously demonstrated that syncytial isopotentiality is a mechanism by which astrocytes function as an efficient system with respect to brain homeostasis. Interestingly, the question of how astrocyte network function changes following chronic stress is yet to be elucidated. Here, we sought to examine the effects of chronic stress on network-level astrocyte (dys)function. Using a transgenic aldh1l1-eGFP astrocyte reporter mouse, a six-week unpredictable chronic mild stress (UCMS) paradigm as a rodent model of major depression, and immunohistochemical approaches, we show that the morphology of individual astrocytes is altered by chronic stress exposure. Additionally, in astrocyte syncytial isopotentiality measurement, we found that UCMS impairs the syncytial coupling strength of astrocytes within the hippocampus and prefrontal cortex—two brain regions that have been implicated in the regulation of mood. Together, these findings reveal that chronic stress leads to astrocyte atrophy and impaired gap junction coupling, raising the prospect that both individual and network-level astrocyte functionality are important in the etiology of major depression and other neuropsychiatric disorders.







Similar content being viewed by others
Data Availability
All data generated or analyzed for this study are included in this article. Please contact the corresponding author with questions or requests.
References
Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, Grant BF (2018) Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiat 75:336–346
Friedrich MJ (2017) Depression Is the leading cause of disability around the world. JAMA 317:1517
Hammen C (2005) Stress and depression. Annu Rev Clin Psychol 1:293–319
Yang L, Zhao Y, Wang Y, Liu L, Zhang X, Li B, Cui R (2015) The Effects of psychological stress on depression. Curr Neuropharmacol 13:494–504
Duman RS (2009) Neuronal damage and protection in the pathophysiology and treatment of psychiatric illness: stress and depression. Dialogues Clin Neurosci 11:239–255
Duman RS, Aghajanian GK (2012) Synaptic dysfunction in depression: potential therapeutic targets. Science 338:68–72
Liu W, Ge T, Leng Y, Pan Z, Fan J, Yang W, Cui R (2017) The role of neural plasticity in depression: from hippocampus to prefrontal cortex. Neural Plast 2017:6871089
Bennett MV, Contreras JE, Bukauskas FF, Saez JC (2003) New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci 26:610–617
Giaume C, McCarthy KD (1996) Control of gap-junctional communication in astrocytic networks. Trends Neurosci 19:319–325
Dermietzel R, Spray DC (1993) Gap junctions in the brain: where, what type, how many and why? Trends Neurosci 16:186–192
Kiyoshi CM, Zhou M (2019) Astrocyte syncytium: a functional reticular system in the brain. Neural Regen Res 14:595–596
Ma B, Buckalew R, Du Y, Kiyoshi CM, Alford CC, Wang W, McTigue DM, Enyeart JJ, Terman D, Zhou M (2016) Gap junction coupling confers isopotentiality on astrocyte syncytium. Glia 64:214–226
Kuga N, Sasaki T, Takahara Y, Matsuki N, Ikegaya Y (2011) Large-scale calcium waves traveling through astrocytic networks in vivo. J Neurosci 31:2607–2614
Langer J, Stephan J, Theis M, Rose CR (2012) Gap junctions mediate intercellular spread of sodium between hippocampal astrocytes in situ. Glia 60:239–252
Lin JH, Weigel H, Cotrina ML, Liu S, Bueno E, Hansen AJ, Hansen TW, Goldman S, Nedergaard M (1998) Gap-junction-mediated propagation and amplification of cell injury. Nat Neurosci 1:494–500
Newman EA (2001) Propagation of intercellular calcium waves in retinal astrocytes and muller cells. J Neurosci 21:2215–2223
Orkand RK, Nicholls JG, Kuffler SW (1966) Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol 29:788–806
Rose CR, Ransom BR (1997) Gap junctions equalize intracellular Na+ concentration in astrocytes. Glia 20:299–307
Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C (2008) Astroglial metabolic networks sustain hippocampal synaptic transmission. Science 322:1551–1555
Simard S, Coppola G, Rudyk CA, Hayley S, McQuaid RJ, Salmaso N (2018) Profiling changes in cortical astroglial cells following chronic stress. Neuropsychopharmacology 43:1961–1971
Wang F, Smith NA, Xu Q, Fujita T, Baba A, Matsuda T, Takano T, Bekar L, Nedergaard M (2012) Astrocytes modulate neural network activity by Ca(2)+-dependent uptake of extracellular K+. Science signaling. https://doi.org/10.1126/scisignal.2002334
Naskar S, Chattarji S (2019) Stress elicits contrasting effects on the structure and number of astrocytes in the amygdala versus hippocampus. eNeuro. https://doi.org/10.1523/ENEURO.0338-18.2019
Tynan RJ, Beynon SB, Hinwood M, Johnson SJ, Nilsson M, Woods JJ, Walker FR (2013) Chronic stress-induced disruption of the astrocyte network is driven by structural atrophy and not loss of astrocytes. Acta Neuropathol 126:75–91
Walker R, Tynan R, Beyon S, Nilsson M (2013) 70. Chronic stress induces profound structural atrophy of astrocytes within the prefrontal cortex: An emerging story in glial remodeling in response to stress. Brain Behav Immun 32:e20–e21
Murphy-Royal C, Johnston AD, Boyce AKJ, Diaz-Castro B, Institoris A, Peringod G, Zhang O, Stout RF, Spray DC, Thompson RJ, Khakh BS, Bains JS, Gordon GR (2020) Stress gates an astrocytic energy reservoir to impair synaptic plasticity. Nat Commun 11:2014
Stephan J, Eitelmann S, Zhou M (2021) Approaches to study gap junctional coupling. Front Cell Neurosci 15:640406
Zhong S, Du Y, Kiyoshi CM, Ma B, Alford CC, Wang Q, Yang Y, Liu X, Zhou M (2016) Electrophysiological behavior of neonatal astrocytes in hippocampal stratum radiatum. Mol Brain 9:34
Kiyoshi CM, Du Y, Zhong S, Wang W, Taylor AT, Xiong B, Ma B, Terman D, Zhou M (2018) Syncytial isopotentiality: A system-wide electrical feature of astrocytic networks in the brain. Glia 66:2756–2769
Yang Y, Vidensky S, Jin L, Jie C, Lorenzini I, Frankl M, Rothstein JD (2011) Molecular comparison of GLT1+ and ALDH1L1+ astrocytes in vivo in astroglial reporter mice. Glia 59:200–207
Logan RW, Edgar N, Gillman AG, Hoffman D, Zhu X, McClung CA (2015) Chronic stress induces brain region-specific alterations of molecular rhythms that correlate with depression-like behavior in mice. Biol Psychiatry 78:249–258
Monteiro S, Roque S, de Sa-Calcada D, Sousa N, Correia-Neves M, Cerqueira JJ (2015) An efficient chronic unpredictable stress protocol to induce stress-related responses in C57BL/6 mice. Front Psychiatry 6:6
Willner P (1997) Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology 134:319–329
Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS (2011) Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69:754–761
Frisbee JC, Brooks SD, Stanley SC, Audiffret AC (2015) An unpredictable chronic mild stress protocol for instigating depressive symptoms, behavioral changes and negative health outcomes in rodents. J Vis Exp. https://doi.org/10.3791/53109
Dominguez G, Henkous N, Prevot T, David V, Guillou JL, Belzung C, Mons N, Beracochea D (2019) Sustained corticosterone rise in the prefrontal cortex is a key factor for chronic stress-induced working memory deficits in mice. Neurobiol Stress 10:100161
Nguyen ET, Selmanovic D, Maltry M, Morano R, Franco-Villanueva A, Estrada CM, Solomon MB (2020) Endocrine stress responsivity and social memory in 3xTg-AD female and male mice: a tale of two experiments. Horm Behav 126:104852
Aten S, Page CE, Kalidindi A, Wheaton K, Niraula A, Godbout JP, Hoyt KR, Obrietan K (2019) miR-132/212 is induced by stress and its dysregulation triggers anxiety-related behavior. Neuropharmacology 144:256–270
Cryan JF, Mombereau C, Vassout A (2005) The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev 29:571–625
Isingrini E, Camus V, Le Guisquet AM, Pingaud M, Devers S, Belzung C (2010) Association between repeated unpredictable chronic mild stress (UCMS) procedures with a high fat diet: a model of fluoxetine resistance in mice. PLoS ONE 5:e10404
Aten S, Kalidindi A, Yoon H, Rumbaugh G, Hoyt KR, Obrietan K (2021) SynGAP is expressed in the murine suprachiasmatic nucleus and regulates circadian-gated locomotor activity and light-entrainment capacity. Eur J Neurosci 53:732–749
Aten S, Hansen KF, Price KH, Wheaton K, Kalidindi A, Garcia A, Alzate-Correa D, Hoyt KR, Obrietan K (2018) miR-132 couples the circadian clock to daily rhythms of neuronal plasticity and cognition. Learn Mem 25:214–229
Aten S, Page CE, Kalidindi A, Wheaton KL, Niraula A, Godbout JP, Hoyt KR, Obrietan K (2018) Data highlighting the expression of two miR-132/212 target genes-Sirt1 and Pten-after chronic stress. Data Brief 21:2323–2329
Huang AY, Woo J, Sardar D, Lozzi B, Bosquez Huerta NA, Lin CJ, Felice D, Jain A, Paulucci-Holthauzen A, Deneen B (2020) Region-specific transcriptional control of astrocyte function oversees local circuit activities. Neuron 106(992–1008):e1009
Lanjakornsiripan D, Pior BJ, Kawaguchi D, Furutachi S, Tahara T, Katsuyama Y, Suzuki Y, Fukazawa Y, Gotoh Y (2018) Layer-specific morphological and molecular differences in neocortical astrocytes and their dependence on neuronal layers. Nat Commun 9:1623
Susaki EA, Tainaka K, Perrin D, Yukinaga H, Kuno A, Ueda HR (2015) Advanced CUBIC protocols for whole-brain and whole-body clearing and imaging. Nat Protoc 10:1709–1727
Susaki EA, Tainaka K, Perrin D, Kishino F, Tawara T, Watanabe TM, Yokoyama C, Onoe H, Eguchi M, Yamaguchi S, Abe T, Kiyonari H, Shimizu Y, Miyawaki A, Yokota H, Ueda HR (2014) Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell 157:726–739
Tainaka K, Kubota SI, Suyama TQ, Susaki EA, Perrin D, Ukai-Tadenuma M, Ukai H, Ueda HR (2014) Whole-body imaging with single-cell resolution by tissue decolorization. Cell 159:911–924
Wang Q, Wang W, Aten S, Kiyoshi CM, Du Y, Zhou M (2020) Epileptiform neuronal discharges impair astrocyte syncytial isopotentiality in acute hippocampal slices. Brain Sci. https://doi.org/10.3390/brainsci10040208
Du Y, Wang W, Lutton AD, Kiyoshi CM, Ma B, Taylor AT, Olesik JW, McTigue DM, Askwith CC, Zhou M (2018) Dissipation of transmembrane potassium gradient is the main cause of cerebral ischemia-induced depolarization in astrocytes and neurons. Exp Neurol 303:1–11
Stephan J, Haack N, Kafitz KW, Durry S, Koch D, Hochstrate P, Seifert G, Steinhauser C, Rose CR (2012) Kir4.1 channels mediate a depolarization of hippocampal astrocytes under hyperammonemic conditions in situ. Glia 60:965–978
Ma BF, Xie MJ, Zhou M (2012) Bicarbonate efflux via GABA(A) receptors depolarizes membrane potential and inhibits two-pore domain potassium channels of astrocytes in rat hippocampal slices. Glia 60:1761–1772
Shepard R, Page CE, Coutellier L (2016) Sensitivity of the prefrontal GABAergic system to chronic stress in male and female mice: Relevance for sex differences in stress-related disorders. Neuroscience 332:1–12
Zhu S, Wang J, Zhang Y, Li V, Kong J, He J, Li XM (2014) Unpredictable chronic mild stress induces anxiety and depression-like behaviors and inactivates AMP-activated protein kinase in mice. Brain Res 1576:81–90
Zhou M, Du Y, Aten S, Terman D (2021) On the electrical passivity of astrocyte potassium conductance. J Neurophysiol 126:1403–1419
Dallerac G, Chever O, Rouach N (2013) How do astrocytes shape synaptic transmission? Insights from electrophysiology. Front Cell Neurosci 7:159
Du Y, Kiyoshi CM, Wang Q, Wang W, Ma B, Alford CC, Zhong S, Wan Q, Chen H, Lloyd EE, Bryan RM (2016) Genetic deletion of TREK-1 or TWIK-1/TREK-1 potassium channels does not alter the basic electrophysiological properties of mature hippocampal astrocytes in situ. Front Cell Neurosci. https://doi.org/10.3389/fncel.2016.00013
Du Y, Ma B, Kiyoshi CM, Alford CC, Wang W, Zhou M (2015) Freshly dissociated mature hippocampal astrocytes exhibit passive membrane conductance and low membrane resistance similarly to syncytial coupled astrocytes. J Neurophysiol 113:3744–3750
Ma B, Xu G, Wang W, Enyeart JJ, Zhou M (2014) Dual patch voltage clamp study of low membrane resistance astrocytes in situ. Mol Brain 7:18
Zhou M, Kimelberg HK (2000) Freshly isolated astrocytes from rat hippocampus show two distinct current patterns and different [K(+)](o) uptake capabilities. J Neurophysiol 84:2746–2757
Huang M, Du Y, Kiyoshi CM, Wu X, Askwith CC, McTigue DM, Zhou M (2018) Syncytial isopotentiality: an electrical feature of spinal cord astrocyte networks. Neuroglia 1:271–279
Ballanyi K, Grafe P, ten Bruggencate G (1987) Ion activities and potassium uptake mechanisms of glial cells in guinea-pig olfactory cortex slices. J Physiol 382:159–174
Hertz L (1965) Possible role of neuroglia: a potassium-mediated neuronal–neuroglial–neuronal impulse transmission system. Nature 206:1091–1094
Newman EA, Frambach DA, Odette LL (1984) Control of extracellular potassium levels by retinal glial cell K+ siphoning. Science 225:1174–1175
Kofuji P, Newman EA (2004) Potassium buffering in the central nervous system. Neuroscience 129:1045–1056
Du Y, Brennan FH, Popovich PG, Zhou M (2022) Microglia maintain the normal structure and function of the hippocampal astrocyte network. Glia 70:1359–1379
Nielsen MS, Axelsen LN, Sorgen PL, Verma V, Delmar M, Holstein-Rathlou NH (2012) Gap junctions. Compr Physiol 2:1981–2035
Verkhratsky A, Lazareva N, Semyanov A (2022) Glial decline and loss of homeostatic support rather than inflammation defines cognitive aging. Neural Regen Res 17:565–566
Popov A, Brazhe A, Denisov P, Sutyagina O, Li L, Lazareva N, Verkhratsky A, Semyanov A (2021) Astrocyte dystrophy in ageing brain parallels impaired synaptic plasticity. Aging Cell 20:e13334
Verkhratsky A, Augusto-Oliveira M, Pivoriunas A, Popov A, Brazhe A, Semyanov A (2021) Astroglial asthenia and loss of function, rather than reactivity, contribute to the ageing of the brain. Pflugers Arch 473:753–774
Izquierdo A, Wellman CL, Holmes A (2006) Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci 26:5733–5738
Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH (2004) Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience 125:1–6
Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH (2009) Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb Cortex 19:2479–2484
Drevets WC, Price JL, Simpson JR Jr, Todd RD, Reich T, Vannier M, Raichle ME (1997) Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386:824–827
Hastings RS, Parsey RV, Oquendo MA, Arango V, Mann JJ (2004) Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology 29:952–959
Chung WS, Allen NJ, Eroglu C (2015) Astrocytes control synapse formation, function, and elimination. Cold Spring Harb Perspect Biol 7:a020370
Ventura R, Harris KM (1999) Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci 19:6897–6906
Semyanov A, Verkhratsky A (2021) Astrocytic processes: from tripartite synapses to the active milieu. Trends Neurosci 44:781–792
Aten S, Kiyoshi CM, Arzola EP, Patterson JA, Taylor AT, Du Y, Guiher AM, Philip M, Camacho EG, Mediratta D, Collins K, Boni K, Garcia SA, Kumar R, Drake AN, Hegazi A, Trank L, Benson E, Kidd G, Terman D, Zhou M (2022) Ultrastructural view of astrocyte arborization, astrocyte-astrocyte and astrocyte-synapse contacts, intracellular vesicle-like structures, and mitochondrial network. Prog Neurobiol 213:102264
Browne CA, Hammack R, Lucki I (2018) Dysregulation of the lateral Habenula in major depressive disorder. Front Synaptic Neurosci 10:46
Campioni MR, Xu M, McGehee DS (2009) Stress-induced changes in nucleus accumbens glutamate synaptic plasticity. J Neurophysiol 101:3192–3198
Zhang X, Ge TT, Yin G, Cui R, Zhao G, Yang W (2018) Stress-induced functional alterations in amygdala: implications for neuropsychiatric diseases. Front Neurosci 12:367
Cerniauskas I, Winterer J, de Jong JW, Lukacsovich D, Yang H, Khan F, Peck JR, Obayashi SK, Lilascharoen V, Lim BK, Foldy C, Lammel S (2019) Chronic Stress Induces Activity, Synaptic, and Transcriptional Remodeling of the Lateral Habenula Associated with Deficits in Motivated Behaviors. Neuron 104(899–915):e898
Miguel-Hidalgo JJ, Moulana M, Deloach PH, Rajkowska G (2018) Chronic Unpredictable Stress Reduces Immunostaining for Connexins 43 and 30 and Myelin Basic Protein in the Rat Prelimbic and Orbitofrontal Cortices. Chronic Stress (Thousand Oaks) 2
Orellana JA, Moraga-Amaro R, Diaz-Galarce R, Rojas S, Maturana CJ, Stehberg J, Saez JC (2015) Restraint stress increases hemichannel activity in hippocampal glial cells and neurons. Front Cell Neurosci 9:102
Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC (2003) Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev 83:1359–1400
Verkhratsky A (2006) Glial calcium signaling in physiology and pathophysiology. Acta Pharmacol Sin 27:773–780
Verkhratsky A, Rodriguez JJ, Parpura V (2012) Calcium signalling in astroglia. Mol Cell Endocrinol 353:45–56
Guerra-Gomes S, Sousa N, Pinto L, Oliveira JF (2017) Functional Roles of Astrocyte Calcium Elevations: From Synapses to Behavior. Front Cell Neurosci 11:427
Torres-Ceja B, Olsen ML (2022) A closer look at astrocyte morphology: Development, heterogeneity, and plasticity at astrocyte leaflets. Curr Opin Neurobiol 74:102550
Aboufares El Alaoui A, Jackson M, Fabri M, de Vivo L, Bellesi M (2020) Characterization of Subcellular Organelles in Cortical Perisynaptic Astrocytes. Front Cell Neurosci 14:573944
Gavrilov N, Golyagina I, Brazhe A, Scimemi A, Turlapov V, Semyanov A (2018) Astrocytic Coverage of Dendritic Spines, Dendritic Shafts, and Axonal Boutons in Hippocampal Neuropil. Front Cell Neurosci 12:248
Patrushev I, Gavrilov N, Turlapov V, Semyanov A (2013) Subcellular location of astrocytic calcium stores favors extrasynaptic neuron-astrocyte communication. Cell Calcium 54:343–349
Acknowledgements
The authors thank Michaela Witt for technical assistance.
Funding
This work was sponsored by a grant from National Institute of Neurological Disorders and Stroke; Grant code: RO1NS116059 (MZ), and an Alumni Grants for Graduate Research and Scholarship (AGGRS) and Distinguished University Fellowship, Ohio State University (to SA).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Conceptualization, data acquisition, data analysis/interpretation, drafting of original manuscript, and manuscript revisions were performed by SA. Conceptualization, data acquisition, data analysis/interpretation, and manuscript revisions were performed by YD. OT, CD, and KC performed data analysis and assisted in manuscript revision. MT assisted with data acquisition and manuscript revision. CK assisted with project conceptualization and manuscript revision. MZ participated in project conceptualization, data interpretation and manuscript revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Animal care protocols and methods were approved by the Ohio State University's Institutional Animal Care and Use Committee (IACUC). Mice were exposed to 2–3 stressors each day for a period of six consecutive weeks. Of note, to prevent animals from habituating to the daily stressors, these stressors were performed at different times throughout the day (i.e., in the morning, afternoon, evening, and overnight).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aten, S., Du, Y., Taylor, O. et al. Chronic Stress Impairs the Structure and Function of Astrocyte Networks in an Animal Model of Depression. Neurochem Res 48, 1191–1210 (2023). https://doi.org/10.1007/s11064-022-03663-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03663-4