Abstract
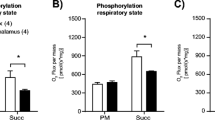
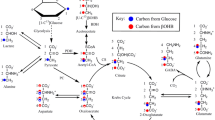
Glucose and oxygen (O2) are vital to the brain. Glucose metabolism and mitochondria play a pivotal role in this process, culminating in the increase of reactive O2 species. Hexokinase (HK) is a key enzyme on glucose metabolism and is coupled to the brain mitochondrial redox modulation by recycling ADP for oxidative phosphorylation (OXPHOS). GABA shunt is an alternative pathway to GABA metabolism that increases succinate levels, a Krebs cycle intermediate. Although glucose and GABA metabolisms are intrinsically connected, their interplay coordinating mitochondrial function is poorly understood. Here, we hypothesize that the HK and the GABA shunt interact to control mitochondrial metabolism differently in the cortex and the hypothalamus. The GABA shunt stimulated mitochondrial O2 consumption and H2O2 production higher in hypothalamic synaptosomes (HSy) than cortical synaptosomes (CSy). The GABA shunt increased the HK coupled to OXPHOS activity in both population of synaptosomes, but the rate of activation was higher in HSy than CSy. Significantly, malonate and vigabatrin blocked the effects of the GABA shunt in the HK activity coupled to OXPHOS. It indicates that the glucose phosphorylation is linked to GABA and Krebs cycle reactions. Together, these data shed light on the HK and SDH role on the metabolism of each region fed by GABA turnover, which depends on the neurons' metabolic route.




Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
Abbreviations
- α-KG :
-
Alpha ketoglutarate
- ΔΨm :
-
Mitochondrial membrane potential
- 2-DG :
-
2-Deoxyglucose
- Ap5A :
-
P1,P5-Di(adenosine-5') pentaphosphate
- CSy :
-
Cortical synaptosome
- ETS :
-
Electron transport system
- FCCP :
-
Carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone
- GABA-t :
-
Gamma transaminase
- GAD :
-
Glutamate decarboxylase
- GDH :
-
Glutamate dehydrogenase
- Glc :
-
Glucose
- G6P :
-
Glucose-6-phosphate
- HK :
-
Hexokinase
- HSy :
-
Hypothalamic synaptosome
- mROS :
-
Mitochondrial reactive oxygen species
- mt-HK :
-
Mitochondrial-bound hexokinase
- NAG :
-
N-Acetyl-Glucosamine
- OXPHOS :
-
Oxidative phosphorylation
- SDH :
-
Succinate dehydrogenase
- SSADH :
-
Succinic semialdehyde dehydrogenase
- Succ :
-
Succinate
- TCA :
-
Tricarboxylic acid cycle
References
Roberts E, Frankel S (1950) Gamma-aminobutyric acid in brain: its formation from glutamic acid. J Biol Chem 187(1):55–63
Curtis DR, Phillis JW, Watkins JC (1959) The depression of spinal neurones by gamma-amino-n-butyric acid and beta-alanine. J Physiol 146(1):185–203
Soghomonian JJ, Martin DL (1998) Two isoforms of glutamate decarboxylase: why? Trends Pharmacol Sci 19(12):500–505
Martin DL, Rimvall K (1993) Regulation of gamma-aminobutyric acid synthesis in the brain. J Neurochem 60(2):395–407
Saito K et al (1974) Immunohistochemical localization of glutamate decarboxylase in rat cerebellum. Proc Natl Acad Sci U S A 71(2):269–273
Walls AB et al (2011) Knockout of GAD65 has major impact on synaptic GABA synthesized from astrocyte-derived glutamine. J Cereb Blood Flow Metab 31(2):494–503
Rowley NM et al (2012) Glutamate and GABA synthesis, release, transport and metabolism as targets for seizure control. Neurochem Int 61(4):546–558
Balazs R et al (1970) The operation of the gamma-aminobutyrate bypath of the tricarboxylic acid cycle in brain tissue in vitro. Biochem J 116(3):445–461
Patel AJ, Balazs R, Richter D (1970) Contribution of the GABA bypath to glucose oxidation, and the development of compartmentation in the brain. Nature 226(5251):1160–1161
Myles WS, Wood JD (1968) The effect of hyperbaric oxygen on the GABA shunt pathway in brain homogenates. Can J Physiol Pharmacol 46(4):669–671
Walsh JM, Clark JB (1976) Studies on the control of 4-aminobutyrate metabolism in “synaptosomal” and free rat brain mitochondria. Biochem J 160(2):147–157
Ravasz D et al (2017) Catabolism of GABA, succinic semialdehyde or gamma-hydroxybutyrate through the GABA shunt impair mitochondrial substrate-level phosphorylation. Neurochem Int 109:41–53
Salminen A et al (2016) Hypoxia and GABA shunt activation in the pathogenesis of Alzheimer’s disease. Neurochem Int 92:13–24
Parviz M et al (2014) Disorders of GABA metabolism: SSADH and GABA-transaminase deficiencies. J Pediatr Epilepsy 3(4):217–227
Chance B, Williams GR (1955) Respiratory enzymes in oxidative phosphorylation I. Kinetics of oxygen utilization. J Biol Chem 217(1):383–93
Mitchell P (1961) Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191:144–148
Vafai SB, Mootha VK (2012) Mitochondrial disorders as windows into an ancient organelle. Nature 491(7424):374–383
Kadenbach B (2003) Intrinsic and extrinsic uncoupling of oxidative phosphorylation. Biochim Biophys Acta 1604(2):77–94
Korshunov SS, Skulachev VP, Starkov AA (1997) High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 416(1):15–18
Havel PJ (2001) Peripheral signals conveying metabolic information to the brain: short-term and long-term regulation of food intake and energy homeostasis. Exp Biol Med (Maywood) 226(11):963–977
Rutter J, Winge DR, Schiffman JD (2010) Succinate dehydrogenase - assembly, regulation and role in human disease. Mitochondrion 10(4):393–401
Mills E, O’Neill LA (2014) Succinate: a metabolic signal in inflammation. Trends Cell Biol 24(5):313–320
Benit P et al (2014) Unsuspected task for an old team: succinate, fumarate and other Krebs cycle acids in metabolic remodeling. Biochim Biophys Acta 1837(8):1330–1337
Tretter L, Patocs A, Chinopoulos C (2016) Succinate, an intermediate in metabolism, signal transduction, ROS, hypoxia, and tumorigenesis. Biochim Biophys Acta 1857(8):1086–1101
Dienel, G.A. 2012 Fueling and imaging brain activation. ASN Neuro 4(5)
Schousboe A (2019) Metabolic signaling in the brain and the role of astrocytes in control of glutamate and GABA neurotransmission. Neurosci Lett 689:11–13
Wilson JE (2003) Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol 206(Pt 12):2049–2057
Santiago AP et al (2008) Reactive oxygen species generation is modulated by mitochondrial kinases: correlation with mitochondrial antioxidant peroxidases in rat tissues. Biochimie 90(10):1566–1577
da Silva WS et al (2004) Mitochondrial bound hexokinase activity as a preventive antioxidant defense: steady-state ADP formation as a regulatory mechanism of membrane potential and reactive oxygen species generation in mitochondria. J Biol Chem 279(38):39846–39855
Camacho-Pereira J et al (2009) Reactive oxygen species production by potato tuber mitochondria is modulated by mitochondrially bound hexokinase activity. Plant Physiol 149(2):1099–1110
Rodrigues-Ferreira C, da Silva AP, Galina A (2012) Effect of the antitumoral alkylating agent 3-bromopyruvate on mitochondrial respiration: role of mitochondrially bound hexokinase. J Bioenerg Biomembr 44(1):39–49
Cavalcanti-de-Albuquerque JP et al (2018) Mitochondria-bound hexokinase (mt-HK) activity differ in cortical and hypothalamic synaptosomes: differential role of mt-HK in H2O2 depuration. Mol Neurobiol 55(7):5889–5900
Hendry SH et al (1987) Numbers and proportions of GABA-immunoreactive neurons in different areas of monkey cerebral cortex. J Neurosci 7(5):1503–1519
Buzsaki G, Kaila K, Raichle M (2007) Inhibition and brain work. Neuron 56(5):771–783
Vincent SR, Hokfelt T, Wu JY (1982) GABA neuron systems in hypothalamus and the pituitary gland. Immunohistochemical demonstration using antibodies against glutamate decarboxylase. Neuroendocrinology 34(2):117–125
Decavel C, Van den Pol AN (1990) GABA: a dominant neurotransmitter in the hypothalamus. J Comp Neurol 302(4):1019–1037
Decavel C, van den Pol AN (1992) Converging GABA- and glutamate-immunoreactive axons make synaptic contact with identified hypothalamic neurosecretory neurons. J Comp Neurol 316(1):104–116
Dunkley PR, Jarvie PE, Robinson PJ (2008) A rapid Percoll gradient procedure for preparation of synaptosomes. Nat Protoc 3(11):1718–1728
Sims NR, Anderson MF (2008) Isolation of mitochondria from rat brain using Percoll density gradient centrifugation. Nat Protoc 3(7):1228–1239
Muller AP et al (2013) Insulin prevents mitochondrial generation of H(2)O(2) in rat brain. Exp Neurol 247:66–72
Gnaiger E (2009) Capacity of oxidative phosphorylation in human skeletal muscle: new perspectives of mitochondrial physiology. Int J Biochem Cell Biol 41(10):1837–1845
Kurebayashi N, Kodama T, Ogawa Y (1980) P1, P5-Di(adenosine-5’)pentaphosphate(Ap5A) as an inhibitor of adenylate kinase in studies of fragmented sarcoplasmic reticulum from bullfrog skeletal muscle. J Biochem 88(3):871–876
Cavalcanti-de-Albuquerque JP et al (2014) Role of estrogen on skeletal muscle mitochondrial function in ovariectomized rats: a time course study in different fiber types. J Appl Physiol 116(7):779–789
de Souza Ferreira E et al (2019) Mitochondria-coupled glucose phosphorylation develops after birth to modulate H2 O2 release and calcium handling in rat brain. J Neurochem 149(5):624–640
Johnson MK (1960) The intracellular distribution of glycolytic and other enzymes in rat-brain homogenates and mitochondrial preparations. Biochem J 77:610–618
Carbone S, Lavie CJ, Arena R (2017) Obesity and Heart Failure: focus on the obesity paradox. Mayo Clin Proc 92(2):266–279
Beattie DS, Sloan HR, Basford RE (1963) Brain mitochondria Ii. The relationship of brain mitochondria to glycolysis. J Cell Biol 19:309–316
Wilson JE (1968) Brain hexokinase. A proposed relation between soluble-particulate distribution and activity in vivo. J Biol Chem 243(13):3640–3647
Crane RK, Sols A (1953) The association of hexokinase with particulate fractions of brain and other tissue homogenates. J Biol Chem 203(1):273–292
BeltrandelRio H, Wilson JE (1991) Hexokinase of rat brain mitochondria: relative importance of adenylate kinase and oxidative phosphorylation as sources of substrate ATP, and interaction with intramitochondrial compartments of ATP and ADP. Arch Biochem Biophys 286(1):183–194
BeltrandelRio H, Wilson JE (1992) Coordinated regulation of cerebral glycolytic and oxidative metabolism, mediated by mitochondrially bound hexokinase dependent on intramitochondrially generated ATP. Arch Biochem Biophys 296(2):667–677
BeltrandelRio H, Wilson JE (1992) Interaction of mitochondrially bound rat brain hexokinase with intramitochondrial compartments of ATP generated by oxidative phosphorylation and creatine kinase. Arch Biochem Biophys 299(1):116–124
de Cerqueira Cesar M, Wilson JE (1995) Application of a double isotopic labeling method to a study of the interaction of mitochondrially bound rat brain hexokinase with intramitochondrial compartments of ATP generated by oxidative phosphorylation. Arch Biochem Biophys 324(1):9–14
de Cerqueira Cesar M, Wilson JE (2002) Functional characteristics of hexokinase bound to the type a and type B sites of bovine brain mitochondria. Arch Biochem Biophys 397(1):106–112
Cesar Mde C, Wilson JE (1998) Further studies on the coupling of mitochondrially bound hexokinase to intramitochondrially compartmented ATP, generated by oxidative phosphorylation. Arch Biochem Biophys 350(1):109–117
Okur V et al (2019) De novo variants in HK1 associated with neurodevelopmental abnormalities and visual impairment. Eur J Hum Genet 27(7):1081–1089
Asadi Shahmirzadi A et al (2020) Alpha-Ketoglutarate, an Endogenous Metabolite, Extends Lifespan and Compresses Morbidity in Aging Mice. Cell Metab 32(3):447–456
Zdzisinska B, Zurek A, Kandefer-Szerszen M (2017) Alpha-ketoglutarate as a molecule with pleiotropic activity: well-known and novel possibilities of therapeutic use. Arch Immunol Ther Exp (Warsz) 65(1):21–36
Brugnara L et al (2012) Metabolomics approach for analyzing the effects of exercise in subjects with type 1 diabetes mellitus. PLoS One 7(7):e40600
Otto C, Yovkova V, Barth G (2011) Overproduction and secretion of alpha-ketoglutaric acid by microorganisms. Appl Microbiol Biotechnol 92(4):689–695
Michaeli S et al (2011) A mitochondrial GABA permease connects the GABA shunt and the TCA cycle, and is essential for normal carbon metabolism. Plant J 67(3):485–498
Brand MD, Chappell JB (1974) Permeability of mitochondria from rat brain and rat liver to GABA. J Neurochem 22(1):47–51
Lai JC, Cooper AJ (1986) Brain alpha-ketoglutarate dehydrogenase complex: kinetic properties, regional distribution, and effects of inhibitors. J Neurochem 47(5):1376–1386
Brown MR, Sullivan PG, Geddes JW (2006) Synaptic mitochondria are more susceptible to Ca2+overload than nonsynaptic mitochondria. J Biol Chem 281(17):11658–11668
Krishnan H, Baquer NZ, Singh R (1981) Changes in gamma-aminobutyric acid-shunt enzymes in regions of rat brain with ketamine anaesthesia. Neuropharmacology 20(6):567–574
Gabellec MM et al (1980) Regional distributions of gamma-aminobutyric acid (GABA), glutamate decarboxylase (GAD), and gamma-aminobutyrate transaminase (GABA-T) in the central nervous brains of C57/BR, C3H/He, and F1 hybrid mice. Neurochem Res 5(3):309–317
Buerstatte CR et al (2000) Brain regional development of the activity of alpha-ketoglutarate dehydrogenase complex in the rat. Brain Res Dev Brain Res 125(1–2):139–145
Gronborg M et al (2010) Quantitative comparison of glutamatergic and GABAergic synaptic vesicles unveils selectivity for few proteins including MAL2, a novel synaptic vesicle protein. J Neurosci 30(1):2–12
Boyken J et al (2013) Molecular profiling of synaptic vesicle docking sites reveals novel proteins but few differences between glutamatergic and GABAergic synapses. Neuron 78(2):285–297
Lassek M, Weingarten J, Volknandt W (2015) The synaptic proteome. Cell Tissue Res 359(1):255–265
Lassek M, Weingarten J, Volknandt W (2014) The Proteome of the Murine Presynaptic Active Zone. Proteomes 2(2):243–257
Volknandt W, Karas M (2012) Proteomic analysis of the presynaptic active zone. Exp Brain Res 217(3–4):449–461
Xu Y et al (2021) Proteomic insights into synaptic signaling in the brain: the past, present and future. Mol Brain 14(1):37
Pedroso AP et al (2019) A proteomics-metabolomics approach indicates changes in hypothalamic glutamate-GABA metabolism of adult female rats submitted to intrauterine growth restriction. Eur J Nutr 58(8):3059–3068
Che-Othman MH et al (2020) Wheat mitochondrial respiration shifts from the tricarboxylic acid cycle to the GABA shunt under salt stress. New Phytol 225(3):1166–1180
Kratz SV (2009) Sensory integration intervention: historical concepts, treatment strategies and clinical experiences in three patients with succinic semialdehyde dehydrogenase (SSADH) deficiency. J Inherit Metab Dis 32(3):353–360
Kim KJ et al (2011) Succinic semialdehyde dehydrogenase: biochemical-molecular-clinical disease mechanisms, redox regulation, and functional significance. Antioxid Redox Signal 15(3):691–718
Koenig MK et al (2017) Phenotype of GABA-transaminase deficiency. Neurology 88(20):1919–1924
Hegde AU et al (2019) GABA Transaminase Deficiency With Survival Into Adulthood. J Child Neurol 34(4):216–220
Schousboe A, Bak LK, Waagepetersen HS (2013) Astrocytic control of biosynthesis and turnover of the neurotransmitters glutamate and GABA. Front Endocrinol (Lausanne) 4:102
Acknowledgements
We would like to thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/Brazil; scholarship number 88887.335745/2019-00 to JPCA), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ/Brazil; grant number E-26/203.004/2017 to AG), Instituto Nacional de Ciência e Tecnologia-Excitotoxicidade e Neuroproteção (INCT-EN) and Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Funding
João Paulo Cavalcanti-de-Albuquerque is currently supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/Brazil; scholarship number 88887.335745/2019–00). Antonio Galina is a fellow from FAPERJ-CNE Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Cientista do Nosso Estado (grant numbers E-26/203.004/2017) and Instituto Nacional de Ciência e Tecnologia-Excitotoxicidade e Neuroproteção (INCT-EN). The study was supported by research grants from CNPq, FAPERJ and CAPES.
Author information
Authors and Affiliations
Contributions
JPCA—Responsible for the development, organization, execution and the writing of the study. ESF—Assisted in animal handling, oxygen consumption and in the hexokinase and citrate synthase activity experiments. DPC—Intellectual contribution. AG—Intellectual contribution and writing.
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interest.
Ethical Approval
The Rio de Janeiro Federal University Institutional Committee for Evaluation of Animal Use in Research (CEUA – CCS – IBCCF179) approved this study, which was in accordance with the International Guiding Principles for Biomedical Research Involving Animals (Geneva, Switzerland).
Consent to Participate
Not applicable.
Consent for Publication
The authors agree with the manuscript publication in case of acceptance.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cavalcanti-de-Albuquerque, J.P., de-Souza-Ferreira, E., de Carvalho, D.P. et al. Coupling of GABA Metabolism to Mitochondrial Glucose Phosphorylation. Neurochem Res 47, 470–480 (2022). https://doi.org/10.1007/s11064-021-03463-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03463-2