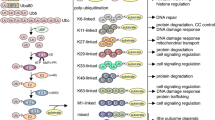
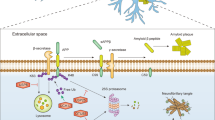
Abstract
Post-translational modification (PTMs) of proteins by ubiquitin and ubiquitin-like modifiers such as interferon-stimulated gene 15 (ISG15) and small ubiquitin-related modifier (SUMO) play a critical role in the regulation of brain pathophysiology. Protein ISGylation is a covalent attachment of ISG15 to its target proteins, which is a unique PTM among other ubiquitin-like modifiers. Although, ISG15 shares sequence homology to ubiquitin, yet the functional significance of protein ISGylation is distinct from ubiquitination and SUMOylation. Further, ISG15 highly conserved among vertebrate species, unlike the other ubiquitin-like modifiers. ISGylation modulates various intracellular mechanisms such as Janus kinase/signal transducers and activators of transcription (JAK-STAT) pathway, autophagy, DNA repair, etc., indicating its biological significance. ISGylation emerged as one of the important mechanisms in the regulation of various neurological disorders including stroke, traumatic brain injury (TBI), basal ganglia calcification, and ataxia-telangiectasia. It appears that protein ISGylation is an endogenous neuroprotective mechanism. This review discusses the role of ISGylation in various brain pathologies with a particular emphasis on cerebral ischemia/stroke and on structural similarities between ISG15 and ubiquitin. Further, recent advancements on the role of ubiquitination and SUMOylation with relevance to ISGylation will also be discussed. The overall goal is to provide better insights on the mechanistic link between ISGylation and other ubiquitin-like modifiers, which may be helpful to establish novel therapeutic strategies for neuroprotection.






Similar content being viewed by others
References
Kerscher O, Felberbaum R, Hochstrasser M (2006) Modification of proteins by ubiquitin and ubiquitin-like proteins. Ann Rev Cell Dev Biol 22:159–180
Ren RJ, Dammer EB, Wang G, Seyfried NT, Levey AI (2014) Proteomics of protein post-translational modifications implicated in neurodegeneration. Transl Neurodegener 3:23
Hershko A, Ciechanover A (1998) The ubiquitin system. Annu Rev Biochem 67:425–479
Braten O et al (2016) Numerous proteins with unique characteristics are degraded by the 26S proteasome following monoubiquitination. Proc Natl Acad Sci USA 113:E4639–E4647
Hallengren J, Chen PC, Wilson SM (2013) Neuronal ubiquitin homeostasis. Cell Biochem Biophys 67:67–73
Zhang D, Zhang DE (2011) Interferon-stimulated gene 15 and the protein ISGylation system. J Interferon Cytokine Res 31(1):119–130
Nakka VP, Lang BT, Lenschow DJ, Zhang DE, Dempsey RJ, Vemuganti R (2011) Increased cerebral protein ISGylation after focal ischemia is neuroprotective. J Cereb Blood Flow Metab 31:2375–2384
Malakhov MP, Malakhova OA, Kim KI, Ritchie KJ, Zhang DE (2002) UBP43 (USP18) specifically removes ISG15 from conjugated proteins. J Biol Chem 277:9976–9981
Fan J-B, Arimoto K-I, Motamedchaboki K, Yan M, Wolf DA, Zhang DE (2015) Identification and characterization of a novel ISG15-ubiquitin mixed chain and its role in regulating protein homeostasis. Sci Rep 5:12704
Loeb KR, Haas AL (1992) The interferon-inducible 15-kDa ubiquitin homolog conjugates to intracellular proteins. J Biol Chem 267:7806–7813
Chang YG, Yan XZ, Xie YY, Gao XC, Song AX, Zhang DE, Hu HY (2008) Different roles for two ubiquitin-like domains of ISG15 in protein modification. J Biol Chem 283:13370–13377
Henley JM, Craig TJ, Wilkinson KA (2014) Neuronal SUMOylation: mechanisms, physiology, and roles in neuronal dysfunction. Physiol Rev 94:1249–1285
Impens F, Radoshevich L, Cossart P, Ribet D (2014) Mapping of SUMO sites and analysis of SUMOylation changes induced by external stimuli. Proc Natl Acad Sci USA 111:12432–12437
Bayer P, Arndt A, Metzger S, Mahajan R, Melchior F, Jaenicke R, Becker J (1998) Structure determination of the small ubiquitin-related modifier SUMO-1. J Mol Biol 280:275–286
Mabb AM, Ehlers MD (2010) Ubiquitination in postsynaptic function and plasticity. Annu Rev Cell Dev Biol 26:179–210
Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR (2000) Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature 404:770–774
Farrell PJ, Broeze RJ, Lengyel P (1979) Accumulation of an mRNA and protein in interferon-treated Ehrlich ascites tumour cells. Nature 279:523–525
Park JH, Yang SW, Park JM, Ka SH, Kim J-H, Kong Y-Y, Jeon YJ, Seol JH, Chung CH (2016) Positive feedback regulation of p53 transactivity by DNA damage-induced ISG15 modification. Nat Commun 7:12513
Villarroya-Beltri C, Guerra S, Madrid S (2017) ISGylation—a key to lock the cell gates for preventing the spread of threats. J Cell Sci 130:2961–2969
Rossi JL, Todd T, Daniels Z, Bazan NG, Belayev L (2015) Interferon-stimulated Gene 15 upregulation precedes the development of blood-brain barrier disruption and cerebral edema after traumatic brain injury in young mice. J Neurotrauma 32:1101–1108
Schwartzenburg J, Juncker M, Reed R, Desai S (2019) Increased ISGylation in cases of TBI-exposed ALS veterans. J Neuropathol Exp Neurol 78:209–218
Ritchie KJ, Malakhov MP, Hetherington CJ, Zhou L, Little MT, Malakhova OA, Sipe JC, Orkin SH, Zhang DE (2002) Dysregulation of protein modification by ISG15 results in brain cell injury. Genes Dev 16:2207–2212
Cimarosti H, Lindberg C, Bomholt SF, Ronn LC, Henley JM (2008) Increased protein SUMOylation following focal cerebral ischemia. Neuropharmacology 54:280–289
Lee YJ, Miyake S, Wakita H, McMullen DC, Azuma Y, Auh S, Hallenbeck JM (2007) Protein SUMOylation is massively increased in hibernation torpor and is critical for the cytoprotection provided by ischemic preconditioning and hypothermia in SHSY5Y cells. J Cereb Blood Flow Metab 27:950–962
Vannucci SJ, Mummery R, Hawkes RB, Rider CC, Beesley PW (1998) Hypoxia-ischemia induces a rapid elevation of ubiquitin conjugate levels and ubiquitin immunoreactivity in the immature rat brain. J Cereb Blood Flow Metab 18:376–385
Yamashita K, Eguchi Y, Kajiwara K, Ito H (1991) Mild hypothermia ameliorates ubiquitin synthesis and prevents delayed neuronal death in the gerbil hippocampus. Stroke 22:1574–1581
Yang W, Sheng H, Warner DS, Paschen W (2008) Transient global cerebral ischemia induces a massive increase in protein sumoylation. J Cereb Blood Flow Metab 28:269–279
Yang W, Sheng H, Homi HM, Warner DS, Paschen W (2008) Cerebral ischemia/stroke and small ubiquitin-like modifier (SUMO) conjugation–a new target for therapeutic intervention? J Neurochem 106:989–999
Albert M, Bécares M, Falqui M, Fernández-Lozano C, Guerra S (2018) ISG15, a small molecule with huge implications: regulation of mitochondrial homeostasis. Viruses 10:629. https://doi.org/10.3390/v10110629
Vijay-Kumar S, Bugg CE, Cook WJ (1987) Structure of ubiquitin refined at 1.8 A resolution. J Mol Biol 194:531–544
Zou W, Zhang DE (2006) The interferon-inducible ubiquitin protein isopeptide ligase (E3) EFP also functions as an ISG15 E3 ligase. J Biol Chem 281:3989–3994
Dastur A, Beaudenon S, Kelley M, Krug RM, Huibregtse JM (2006) Herc5, an interferon-induced HECT E3 enzyme, is required for conjugation of ISG15 in human cells. J Biol Chem 281:4334–4338
Lenschow DJ, Lai C, Frias-Staheli N, Giannakopoulos NV, Lutz A, Wolff T, Osiak A, Levine B, Schmidt RE, Garcia-Sastre A, Leib DA, Pekosz A, Knobeloch KP, Horak I, Virgin HW (2007) IFN-stimulated gene 15 functions as a critical antiviral molecule against influenza, herpes, and Sindbis viruses. Proc Natl Acad Sci USA 104:1371–1376
Liu M, Li XL, Hassel BA (2003) Proteasomes modulate conjugation to the ubiquitin-like protein, ISG15. J Biol Chem 278:1594–1602
Malakhov MP, Kim KI, Malakhova OA, Jacobs BS, Borden EC, Zhang DE (2003) High-throughput immunoblotting. Ubiquitiin-like protein ISG15 modifies key regulators of signal transduction. J Biol Chem 278:16608–16613
Malakhova OA, Yan M, Malakhov MP, Yuan Y, Ritchie KJ, Kim KI, Peterson LF, Shuai K, Zhang DE (2003) Protein ISGylation modulates the JAK-STAT signaling pathway. Genes Dev 17:455–460
Anheim M, Tranchant C, Koenig M (2012) The autosomal recessive cerebellar ataxias. N Engl J Med 366:636–646
Tal E, Alfo M, Zha S, Barzilai A, De Zeeuw CI, Ziv Y, Shiloh Y (2018) Inactive Atm abrogates DSB repair in mouse cerebellum more than does Atm loss, without causing a neurological phenotype. DNA Repair (Amst) 72:10–17
Wood LM, Sankar S, Reed RE, Haas AL, Liu LF, McKinnon P, Desai SD (2011) A novel role for ATM in regulating proteasome-mediated protein degradation through suppression of the ISG15 conjugation pathway. PLoS ONE 6(1):16422
Desai SD, Reed RE, Babu S, Lorio EA (2013) ISG15 deregulates autophagy in genotoxin-treated ataxia telangiectasia cells. J Biol Chem 288:2388–2402
Kim CD, Reed RE, Juncker MA, Fang Z, Desai SD (2017) Evidence for the deregulation of protein turnover pathways in Atm-deficient mouse cerebellum: an organotypic study. J Neuropathol Exp Neurol 76:578–584
Borsini A, Cattaneo A, Malpighi C, Thuret S, Harrison NA; MRC ImmunoPsychiatry Consortium, Zunszain PA, Pariante CM (2018) Interferon-alpha reduces human hippocampal neurogenesis and increases apoptosis via activation of distinct STAT1-dependent mechanisms. Int J Neuropsychopharmacol 21:187–200
Reich N, Evans B, Levy D, Fahey D, Knight E Jr, Darnell JE Jr (1987) Interferon-induced transcription of a gene encoding a 15-kDa protein depends on an upstream enhancer element. Proc Natl Acad Sci USA 84:6394–6398
Knight E Jr, Fahey D, Cordova B, Hillman M, Kutny R, Reich N, Blomstrom D (1988) A 15-kDa interferon-induced protein is derived by COOH-terminal processing of a 17-kDa precursor. J Biol Chem 263:4520–4522
Narasimhan J, Wang M, Fu Z, Klein JM, Haas AL, Kim JJ (2005) Crystal structure of the interferon-induced ubiquitin-like protein ISG15. J Biol Chem 280:27356–27365
Bogunovic D, Boisson-Dupuis S, Casanova JL (2013) ISG15: leading a double life as a secreted molecule. Exp Mol Med. https://doi.org/10.1038/emm.2013.36
Dzimianski JV, Scholte FEM, Bergeron É, Pegan SD (2019) ISG15: it's complicated. J Mol Biol 431:4203–4216
Swaim CD, Scott AF, Canadeo LA, Huibregtse JM (2017) Extracellular ISG15 signals cytokine secretion through the LFA-1 integrin receptor. Mol Cell 68:581–590
Zhang Y, Thery F, Wu NC et al (2019) The in vivo ISGylome links ISG15 to metabolic pathways and autophagy upon Listeria monocytogenes infection. Nat Commun 10:5383
Nakka VP, Gusain A, Mehta SL, Raghubir R (2008) Molecular mechanisms of apoptosis in cerebral ischemia: multiple neuroprotective opportunities. Mol Neurobiol 37:7–38
Nakka VP, Prakash-Babu P, Vemuganti R (2016) Crosstalk between endoplasmic reticulum stress, oxidative stress, and autophagy: potential therapeutic targets for acute CNS injuries. Mol Neurobiol 53:532–544
Bogunovic et al (2012) Mycobacterial disease and impaired IFN-γ immunity in humans with inherited ISG15 deficiency. Science 337:1684–1688
Zhang X et al (2015) Human intracellular ISG15 prevents interferon-alpha/beta over-amplification and auto-inflammation. Nature 517:89–93
Ren-Gang W, Marcus K, Dong-Xian Z (2012) Interferon-stimulated gene 15 as a general marker for acute and chronic neuronal injuries. Sheng Li Xue Bao 64:577–583
Wang R, Yang B, Zhang D (2011) Activation of interferon signaling pathways in spinal cord astrocytes from an ALS mouse model. Glia 59:946–958
Vinsant S et al (2013) Characterization of early pathogenesis in the SOD1 (G93A) mouse model of ALS: part II, results and discussion. Brain Behav 3:431–457
Durfee LA, Lyon N, Seo K, Huibregtse JM (2010) The ISG15 conjugation system broadly targets newly synthesized proteins: implications for the antiviral function of ISG15. Mol Cell 38:722–732
Hay RT (2005) SUMO: a history of modification. Mol Cell 18:1–12
Sampson DA, Wang M, Matunis MJ (2001) The small ubiquitin-like modifier-1 (SUMO-1) consensus sequence mediates Ubc9 binding and is essential for SUMO-1 modification. J Biol Chem 276:21664–21669
Yeh ET (2009) SUMOylation and De-SUMOylation: wrestling with life's processes. J Biol Chem 284:8223–8227
Silveirinha V, Stephens GJ, Cimarosti H (2013) Molecular targets underlying SUMO-mediated neuroprotection in brain ischemia. J Neurochem 127:580–591
Zhang L, Liu X, Sheng H, Liu S, Li Y, Zhao JQ, Warner DS, Paschen W, Yang W (2017) Neuron-specific SUMO knockdown suppresses global gene expression response and worsens functional outcome after transient forebrain ischemia in mice. Neuroscience 343:190–212
Wilkinson KA, Henley JM (2010) Mechanisms, regulation and consequences of protein SUMOylation. Biochem J 428:133–145
Yang W, Sheng H, Thompson JW et al (2014) Small ubiquitin-like modifier 3-modified proteome regulated by brain ischemia in novel small ubiquitin-like modifier transgenic mice: putative protective proteins/pathways. Stroke 45:1115–1122
Lee YJ, Castri P, Bembry J, Maric D, Auh S, Hallenbeck JM (2009) SUMOylation participates in induction of ischemic tolerance. J Neurochem 109:257–267
Datwyler AL, Lättig-Tünnemann G, Yang W, Paschen W, Lee SL, Dirnagl U, Endres M, Harms C (2011) SUMO2/3 conjugation is an endogenous neuroprotective mechanism. J Cereb Blood Flow Metab 31:21529
Lee YJ, Mou Y, Maric D, Klimanis D, Auh S, Hallenbeck JM (2011) Elevated global SUMOylation in Ubc9 transgenic mice protects their brains against focal cerebral ischemic damage. PLoS ONE 6:e25852
Zhang H, Wang Y, Zhu A, Huang D, Deng S, Cheng J, Zhu MX, Li Y (2016) SUMO-specific protease 1 protects neurons from apoptotic death during transient brain ischemia/reperfusion. Cell Death Dis 7(11):e2484
Vertegaal AC, Andersen JS, Ogg SC, Hay RT, Mann M, Lamond AI (2006) Distinct and overlapping sets of SUMO-1 and SUMO-2 target proteins revealed by quantitative proteomics. Mol Cell Proteomics 5:2298–2310
Wagner SA, Beli P, Weinert BT, Nielsen ML, Cox J, Mann M, Choudhary C (2011) proteome-wide, quantitative survey of in vivo ubiquitylation sites reveals widespread regulatory roles. Mol Cell Proteomics 10(M111):013284
Dennissen FJ, Kholod N, van Leeuwen FW (2012) The ubiquitin proteasome system in neurodegenerative diseases: culprit, accomplice or victim? Prog Neurobiol 96:190–207
Glickman MH, Ciechanover A (2002) The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82:373–428
Ge P, Luo Y, Liu CL, Hu B (2007) Protein aggregation and proteasome dysfunction after brain ischemia. Stroke 38:3230–3236
Hayashi T, Takada K, Matsuda M (1992) Post-transient ischemia increase in ubiquitin conjugates in the early reperfusion. NeuroReport 3:519–520
Hu BR, Janelidze S, Ginsberg MD, Busto R, Perez-Pinzon M, Sick TJ, Siesjö BK, Liu CL (2001) Protein aggregation after focal brain ischemia and reperfusion. J Cereb Blood Flow Metab 21:865–875
Lai E, Teodoro T, Volchuk A (2007) Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology 22:193–201
Paschen W, Gissel C, Linden T, Althausen S, Doutheil J (1998) Activation of gadd153 expression through transient cerebral ischemia: evidence that ischemia causes endoplasmic reticulum dysfunction. Brain Res Mol Brain Res 60:115–122
Nakka VP, Gusain A, Raghubir R (2010) Endoplasmic reticulum stress plays critical role in brain damage after cerebral ischemia/reperfusion in rats. Neurotox Res 17:189–202
Hochrainer K, Jackman K, Benakis C, Anrather J, Iadecola C (2015) SUMO2/3 is associated with ubiquitinated protein aggregates in the mouse neocortex after middle cerebral artery occlusion. J Cereb Blood Flow Metab 35:1–5
Liebelt F, Vertegaal AC (2016) Ubiquitin-dependent and independent roles of SUMO in proteostasis. Am J Physiol Cell Physiol 311:C284–C296
Papouli E, Chen S, Davies AA, Huttner D, Krejci L, Sung P, Ulrich HD (2005) Crosstalk between SUMO and ubiquitin on PCNA is mediated by recruitment of the helicase Srs2p. Mol Cell 19:123–133
Gali H, Juhasz S, Morocz M, Hajdu I, Fatyol K, Szukacsov V, Burkovics P, Haracska L (2012) Role of SUMO modification of human PCNA at stalled replication fork. Nucleic Acids Res 40:6049–6059
Malakhova OA, Zhang DE (2008) ISG15 inhibits Nedd4 ubiquitin E3 activity and enhances the innate antiviral response. J Biol Chem 283:8783–8787
Pérez Berrocal DA, Witting KF, Ovaa H, Mulder MPC (2020) Hybrid chains: a collaboration of ubiquitin and ubiquitin-like modifiers introducing cross-functionality to the ubiquitin code. Front Chem 7:931
Kumar R, González-Prieto R, Xiao Z, Verlaan-de Vries M, Vertegaal ACO (2017) The STUbL RNF4 regulates protein group SUMOylation by targeting the SUMO conjugation machinery. Nat Commun 8:1809
Armstrong AA, Mohideen F, Lima CD (2012) Recognition of SUMO-modified PCNA requires tandem receptor motifs in Srs2. Nature 483:59–63
Lamoliatte F, McManus FP, Maarifi G, Chelbi-Alix MK, Thibault P (2017) Uncovering the SUMOylation and ubiquitylation crosstalk in human cells using sequential peptide immunopurification. Nat Commun 8:14109
Yoo L, Yoon AR, Yun CO, Chung KC (2018) Covalent ISG15 conjugation to CHIP promotes its ubiquitin E3 ligase activity and inhibits lung cancer cell growth in response to type I interferon. Cell Death Dis 9:97
Acknowledgements
We sincerely thank science and engineering research board (SERB/EEQ/2017/000804) and University Grants Commission (UGC) faculty recharge program, Government of India. We also thank Prof. Raghu Vemuganti, department of neurological surgery, UW-Madison, the USA for assistance, advice, and discussions.
Funding
Authors received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
This is a review article; therefore does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nakka, V.P., Mohammed, A.Q. A Critical Role for ISGylation, Ubiquitination and, SUMOylation in Brain Damage: Implications for Neuroprotection. Neurochem Res 45, 1975–1985 (2020). https://doi.org/10.1007/s11064-020-03066-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-03066-3