Abstract
Objectives
Neurocytomas (NCs) are rare intracranial tumors that can often be surgically resected. However, disease course is unpredictable in many patients and medical therapies are lacking. We have used whole exome sequencing to explore the molecular etiology for neurocytoma and assist in target identification to develop novel therapeutic interventions.
Methods
We used whole exome sequencing (WES) to compare the molecular landscape of 21 primary & recurrent NCs to five normal cerebellar control samples. WES data was analyzed using the Qiagen Clinical Insight program, variants of interest (VOI) were interrogated using ConSurf, ScoreCons, & Ingenuity Pathway Analysis Software to predict their potential functional effects, and Copy number variations (CNVs) in the genes of interest were analyzed by Genewiz (Azenta Life Sciences).
Results
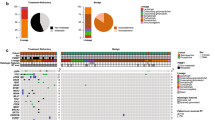
Of 40 VOI involving thirty-six genes, 7 were pathogenic, 17 likely-pathogenic, and 16 of uncertain-significance. Of seven pathogenic NC associated variants, Glucosylceramidase beta 1 [GBA1 c.703T > C (p.S235P)] was mutated in 5/21 (24%), Coagulation factor VIII [F8 c.3637dupA (p.I1213fs*28)] in 4/21 (19%), Phenylalanine hydroxylase [PAH c.975C > A (p.Y325*)] in 3/21 (14%), and Fanconi anemia complementation group C [FANCC c.1162G > T (p.G388*)], Chromodomain helicase DNA binding protein 7 [CHD7 c.2839C > T (p.R947*)], Myosin VIIA [MYO7A c.940G > T (p.E314*)] and Dynein axonemal heavy chain 11 [DNAH11 c.3544C > T (p.R1182*)] in 2/21 (9.5%) NCs respectively. CNVs were noted in 85% of these latter 7 genes. Interestingly, a Carboxy-terminal domain RNA polymerase II polypeptide A small phosphatase 2 [CTDSP2 c.472G > A (p.E158K)] of uncertain significance was also found in > 70% of NC cases.
Interpretation
The variants of interest we identified in the NCs regulate a variety of neurological processes including cilia motility, cell metabolism, immune responses, and DNA damage repair and provide novel insights into the molecular pathogenesis of these extremely rare tumors.
Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- CNC:
-
Central neurocytoma
- CNV:
-
Copy number variation
- ENC:
-
Extra-ventricular neurocytoma
- FFPE:
-
Formalin-Fixed Paraffin-Embedded
- gDNA:
-
Genomic DNA
- GOI:
-
Gene(s) of interest
- VOI:
-
Variant(s) of interest
- VUS:
-
Variant(s) of uncertain significance
- NC:
-
Neurocytoma
- NCv:
-
NC-associated variants
- NT:
-
Normal tissues
- NA:
-
Not available
- SNVs:
-
Single nucleotide variations
- WES:
-
Whole exome sequencing
References
Zacharoulis S, Morales La Madrid A, Bandopadhayay P, Chi SN, Manley P, Ullrich NN, Marcus K, Goumnerova L, Scott RM, Proctor M, Young-Poussaint T, De Girolami U, Kieran MW (2016) Central Versus Extraventricular Neurocytoma in children: a Clinicopathologic Comparison and review of the literature. J Pediatr Hematol Oncol 38:479–485. https://doi.org/10.1097/mph.0000000000000627
Han L, Niu H, Wang J, Wan F, Shu K, Ke C, Lei T (2013) Extraventricular neurocytoma in pediatric populations: a case report and review of the literature. Oncol Lett 6:1397–1405. https://doi.org/10.3892/ol.2013.1583
Piras M, Miele E, Di Giannatale A, Colafati GS, Diomedi-Camassei F, Vinci M, de Billy E, Mastronuzzi A, Carai A (2018) Congenital Extra-Ventricular (Ganglio)Neurocytoma of the Brain Stem: A Case Report. Frontiers in Pediatrics 6
Ajithkumar T, Imbulgoda N, Rees E, Harris F, Horan G, Burke A, Jefferies S, Price S, Cross J, Allinson K (2019) Uncommon low-grade brain tumors. Neurooncology 21:151–166. https://doi.org/10.1093/neuonc/noy151
Kim CH, Jung HW, Hwang SG, Chi JG (2003) Cerebral central neurocytoma with high proliferative index: Case Report. J Korean Neurosurg Soc 33:195–198
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109. https://doi.org/10.1007/s00401-007-0243-4
Han S, Yang Z, Yang Y, Qi X, Yan C, Yu C (2020) Individual Treatment Decisions for Central Neurocytoma. Front Neurol 11. https://doi.org/10.3389/fneur.2020.00834
Kojima K, Arakawa Y, Takeuchi Y, Terada Y, Tanji M, Mineharu Y, Haga H, Miyamoto S (2022) Malignant transformation of central neurocytoma with dissemination 17 years after initial treatment: illustrative case. Journal of Neurosurgery: Case Lessons 3
Kojima K, Arakawa Y, Takeuchi Y, Terada Y, Tanji M, Mineharu Y, Haga H, Miyamoto S (2022) Malignant transformation of central neurocytoma with dissemination 17 years after initial treatment: illustrative case. J Neurosurgery: Case Lessons 3:CASE21610. https://doi.org/10.3171/CASE21610
Mozes P, Szanto E, Tiszlavicz L, Barzo P, Cserhati A, Fodor E, Hideghety K (2014) Clinical course of central neurocytoma with malignant transformation-an indication for craniospinal irradiation. Pathol Oncol Res 20:319–325. https://doi.org/10.1007/s12253-013-9697-y
Mahavadi AK, Patel PM, Kuchakulla M, Shah AH, Eichberg D, Luther EM, Komotar RJ, Ivan ME (2020) Central Neurocytoma Treatment modalities: a systematic review assessing the outcomes of combined maximal safe resection and Radiotherapy with Gross Total Resection. World Neurosurg 137:e176–e182. https://doi.org/10.1016/j.wneu.2020.01.114
Imber BS, Braunstein SE, Wu FY, Nabavizadeh N, Boehling N, Weinberg VK, Tihan T, Barnes M, Mueller S, Butowski NA, Clarke JL, Chang SM, McDermott MM, Prados MD, Berger MS, Haas-Kogan DA (2016) Clinical outcome and prognostic factors for central neurocytoma: twenty year institutional experience. J Neurooncol 126:193–200. https://doi.org/10.1007/s11060-015-1959-y
Fischer J, Pohl A, Volland R, Hero B, Dübbers M, Cernaianu G, Berthold F, von Schweinitz D, Simon T (2017) Complete surgical resection improves outcome in INRG high-risk patients with localized neuroblastoma older than 18 months. BMC Cancer 17:520–520. https://doi.org/10.1186/s12885-017-3493-0
Makale MT, McDonald CR, Hattangadi-Gluth JA, Kesari S (2017) Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat Rev Neurol 13:52–64. https://doi.org/10.1038/nrneurol.2016.185
Wang W, Corominas R, Lin GN (2019) De novo mutations from whole exome sequencing in Neurodevelopmental and Psychiatric disorders: from Discovery to Application. Front Genet 10. https://doi.org/10.3389/fgene.2019.00258
Arts P, Simons A, AlZahrani MS, Yilmaz E, AlIdrissi E, van Aerde KJ, Alenezi N, AlGhamdi HA, AlJubab HA, Al-Hussaini AA, AlManjomi F, Alsaad AB, Alsaleem B, Andijani AA, Asery A, Ballourah W, Bleeker-Rovers CP, van Deuren M, van der Flier M, Gerkes EH, Gilissen C, Habazi MK, Hehir-Kwa JY, Henriet SS, Hoppenreijs EP, Hortillosa S, Kerkhofs CH, Keski-Filppula R, Lelieveld SH, Lone K, MacKenzie MA, Mensenkamp AR, Moilanen J, Nelen M, ten Oever J, Potjewijd J, van Paassen P, Schuurs-Hoeijmakers JHM, Simon A, Stokowy T, van de Vorst M, Vreeburg M, Wagner A, van Well GTJ, Zafeiropoulou D, Zonneveld-Huijssoon E, Veltman JA, van Zelst-Stams WAG, Faqeih EA, van de Veerdonk FL, Netea MG, Hoischen A (2019) Exome sequencing in routine diagnostics: a generic test for 254 patients with primary immunodeficiencies. Genome Med 11:38. https://doi.org/10.1186/s13073-019-0649-3
Flannick J, Mercader JM, Fuchsberger C, Udler MS, Mahajan A, Wessel J, Teslovich TM, Caulkins L, Koesterer R, Barajas-Olmos F, Blackwell TW, Boerwinkle E, Brody JA, Centeno-Cruz F, Chen L, Chen S, Contreras-Cubas C, C?rdova E, Correa A, Cortes M, DeFronzo RA, Dolan L, Drews KL, Elliott A, Floyd JS, Gabriel S, Garay-Sevilla ME, Garc?a-Ortiz H, Gross M, Han S, Heard-Costa NL, Jackson AU, J?rgensen ME, Kang HM, Kelsey M, Kim B-J, Koistinen HA, Kuusisto J, Leader JB, Linneberg A, Liu C-T, Liu J, Lyssenko V, Manning AK, Marcketta A, Malacara-Hernandez JM, Mart?nez-Hern?ndez A, Matsuo K, Mayer-Davis E, Mendoza-Caamal E, Mohlke KL, Morrison AC, Ndungu A, Ng MCY, O?Dushlaine C, Payne AJ, Pihoker C, Post WS, Preuss M, Psaty BM, Vasan RS, Rayner NW, Reiner AP, Revilla-Monsalve C, Robertson NR, Santoro N, Schurmann C, So WY, Sober?n X, Stringham HM, Strom TM, Tam CHT, Thameem F, Tomlinson B, Torres JM, Tracy RP, van Dam RM, Vujkovic M, Wang S, Welch RP, Witte DR, Wong T-Y, Atzmon G, Barzilai N, Blangero J, Bonnycastle LL, Bowden DW, Chambers JC, Chan E, Cheng C-Y, Cho YS, Collins FS, de Vries PS, Duggirala R, Glaser B, Gonzalez C, Gonzalez ME, Groop L, Kooner JS, Kwak SH, Laakso M, Lehman DM, Nilsson P, Spector TD, Tai ES, Tuomi T, Tuomilehto J, Wilson JG, Aguilar-Salinas CA, Bottinger E, Burke B, Carey DJ, Chan JCN, Dupuis J, Frossard P, Heckbert SR, Hwang MY, Kim YJ, Kirchner HL, Lee J-Y, Lee J, Loos RJF, Ma RCW, Morris AD, O?Donnell CJ, Palmer CNA, Pankow J, Park KS, Rasheed A, Saleheen D, Sim X, Small KS, Teo YY, Haiman C, Hanis CL, Henderson BE, Orozco L, Tusi?-Luna T, Dewey FE, Baras A, Gieger C, Meitinger T, Strauch K, Lange L, Grarup N, Hansen T, Pedersen O, Zeitler P, Dabelea D, Abecasis G, Bell GI, Cox NJ, Seielstad M, Sladek R, Meigs JB, Rich SS, Rotter JI, Altshuler D, Burtt NP, Scott LJ, Morris AP, Florez JC, McCarthy MI, Boehnke M, Broad Genomics P, Discov EHRC, Charge, LuCamp, ProDiGy, GoT2D, Esp, Sigma TD, T2D G, Amp T2D G (2019) Exome sequencing of 20,791 cases of type 2 diabetes and 24,440 controls. Nature 570:71–76. https://doi.org/10.1038/s41586-019-1231-2
Ben Chorin A, Masrati G, Kessel A, Narunsky A, Sprinzak J, Lahav S, Ashkenazy H, Ben-Tal N (2020) ConSurf-DB: an accessible repository for the evolutionary conservation patterns of the majority of PDB proteins. Protein Sci 29:258–267. https://doi.org/10.1002/pro.3779
Valdar WS (2002) Scoring residue conservation. Proteins Struct Funct Bioinform 48:227–241
Xiao F, Luo X, Hao N, Niu YS, Xiao X, Cai G, Amos CI, Zhang H (2019) An accurate and powerful method for copy number variation detection. Bioinformatics 35:2891–2898. https://doi.org/10.1093/bioinformatics/bty1041
Sahajpal N, Mondal A, Ahluwalia M, Njau A, Kolhe R (2020) Abstract 5482: validation of somatic variant interpretations from comprehensive cancer panels using QIAGEN Clinical Insight Interpret. Cancer Res 80:5482–5482. https://doi.org/10.1158/1538-7445.AM2020-5482
Balasar Ö, Başdemirci M (2023) Assessment of whole-exome sequencing results in neurogenetic diseases. J Hum Genet: 1–8
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Sci 17:405–423
Surface M, Balwani M, Waters C, Haimovich A, Gan-Or Z, Marder KS, Hsieh T, Song L, Padmanabhan S, Hsieh F, Merchant KM, Alcalay RN (2022) Plasma glucosylsphingosine in GBA1 mutation carriers with and without Parkinson’s Disease. Mov Disorders: Official J Mov Disorder Soc 37:416–421. https://doi.org/10.1002/mds.28846
Kim S, Yun SP, Lee S, Umanah GE, Bandaru VVR, Yin X, Rhee P, Karuppagounder SS, Kwon S-H, Lee H, Mao X, Kim D, Pandey A, Lee G, Dawson VL, Dawson TM, Ko HS (2018) GBA1 deficiency negatively affects physiological α-synuclein tetramers and related multimers. Proceedings of the National Academy of Sciences 115: 798–803 https://doi.org/10.1073/pnas.1700465115
Avenali M, Blandini F, Cerri S (2020) Glucocerebrosidase defects as a major risk factor for Parkinson’s Disease. Front Aging Neurosci 12. https://doi.org/10.3389/fnagi.2020.00097
Ryan E, Jong T, Sidransky E (2022) Newborn screening in Gaucher Disease: a Bright and Complicated Future. OBM Genet 06:165. https://doi.org/10.21926/obm.genet.2203165
Lunghi G, Carsana EV, Loberto N, Cioccarelli L, Prioni S, Mauri L, Bassi R, Duga S, Straniero L, Asselta R (2022) β-Glucocerebrosidase Deficiency activates an aberrant lysosome-plasma membrane Axis responsible for the Onset of Neurodegeneration. Cells 11:2343
Venceslá A, Corral-Rodríguez MÁ, Baena M, Cornet M, Domènech M, Baiget M, Fuentes-Prior P, Tizzano EF (2008) Identification of 31 novel mutations in the F8 gene in Spanish hemophilia a patients: structural analysis of 20 missense mutations suggests new intermolecular binding sites. Blood 111:3468–3478. https://doi.org/10.1182/blood-2007-08-108068
Wang J, Gu J, Chen H, Wu Q, Xiong L, Qiao B, Zhang Y, Xiao H, Tong Y (2022) A Novel Deletion Mutation of the F8 Gene for Hemophilia A. Diagnostics
Al-Huniti A, Harshman LA, Novak M, Nopoulos P, Staber JM (2019) Brain anomalies in children with severe factor VIII Deficiency- a pilot study. Blood 134:1121–1121. https://doi.org/10.1182/blood-2019-131804
Zanon E, Manara R, Milan M, Brandolin B, Mapelli D, Mardari R, Rosini S, Amodio P (2014) Cognitive dysfunctions and cerebral microbleeds in adult patients with haemophilia A: a clinical and MRI pilot-study. Thromb Res 134:851–855. https://doi.org/10.1016/j.thromres.2014.08.005
Flydal MI, Martinez A (2013) Phenylalanine hydroxylase: function, structure, and regulation. IUBMB Life 65:341–349. https://doi.org/10.1002/iub.1150
Roberts KM, Khan CA, Hinck CS, Fitzpatrick PF (2014) Activation of phenylalanine hydroxylase by Phenylalanine does not require binding in the active site. Biochemistry 53:7846–7853. https://doi.org/10.1021/bi501183x
van Spronsen FJ, Hoeksma M, Reijngoud DJ (2009) Brain dysfunction in phenylketonuria: is phenylalanine toxicity the only possible cause? J Inherit Metab Dis 32:46–51. https://doi.org/10.1007/s10545-008-0946-2
Feng W, Shao C, Liu H-K (2017) Versatile roles of the chromatin remodeler CHD7 during Brain Development and Disease. Front Mol Neurosci 10. https://doi.org/10.3389/fnmol.2017.00309
Lettieri A, Oleari R, Paganoni AJJ, Gervasini C, Massa V, Fantin A, Cariboni A (2021) Semaphorin Regulation by the chromatin remodeler CHD7: an Emerging Genetic Interaction shaping neural cells and neural crest in Development and Cancer. Front cell Dev Biology 9:638674–638674. https://doi.org/10.3389/fcell.2021.638674
Kohmoto T, Shono M, Naruto T, Watanabe M, Suga K-i, Nakagawa R, Kagami S, Masuda K, Imoto I (2016) A novel frameshift mutation of CHD7 in a Japanese patient with CHARGE syndrome. Hum Genome Variation 3:16004. https://doi.org/10.1038/hgv.2016.4
Reddy NC, Majidi SP, Kong L, Nemera M, Ferguson CJ, Moore M, Goncalves TM, Liu H-K, Fitzpatrick JAJ, Zhao G, Yamada T, Bonni A, Gabel HW (2021) CHARGE syndrome protein CHD7 regulates epigenomic activation of enhancers in granule cell precursors and gyrification of the cerebellum. Nat Commun 12:5702. https://doi.org/10.1038/s41467-021-25846-3
Shahid M, Azfaralariff A, Zubair M, Abdulkareem Najm A, Khalili N, Law D, Firasat S, Fazry S (2022) In silico study of missense variants of FANCA, FANCC and FANCG genes reveals high risk deleterious alleles predisposing to fanconi anemia pathogenesis. Gene 812:146104. https://doi.org/10.1016/j.gene.2021.146104
Xia M, Li X, Ye S, Zhang Q, Zhao T, Li R, Zhang Y, Xian M, Li T, Li H (2022) FANCC deficiency mediates microglial pyroptosis and secondary neuronal apoptosis in spinal cord contusion. Cell & Bioscience 12:1–15
Zhang X, Li J, Sejas DP, Rathbun KR, Bagby GC, Pang Q (2004) The Fanconi Anemia proteins functionally interact with the protein kinase regulated by RNA (PKR) *. J Biol Chem 279:43910–43919. https://doi.org/10.1074/jbc.M403884200
Huang F, Ben Aissa M, Lévesque G, Carreau M (2018) FANCC localizes with UNC5A at neurite outgrowth and promotes neuritogenesis. BMC Res Notes 11:662. https://doi.org/10.1186/s13104-018-3763-1
Huang F, Ben Aissa M, Magron A, Huard CC, Godin C, Lévesque G, Carreau M (2014) The Fanconi Anemia Group C Protein Interacts with uncoordinated 5A and delays apoptosis. PLoS ONE 9:e92811. https://doi.org/10.1371/journal.pone.0092811
Merfort LW, Lisboa MD, Cavalli LR, Bonfim CM (2022) Cytogenetics in Fanconi Anemia: the Importance of Follow-Up and the search for new biomarkers of genomic instability. Int J Mol Sci.
Rhee DB, Wang Y, Mizesko M, Zhou F, Haneline L, Liu Y (2010) FANCC suppresses short telomere-initiated telomere sister chromatid exchange. Hum Mol Genet 19:879–887
Gillespie PG, Walker RG (2001) Molecular basis of mechanosensory transduction. Nature 413:194–202. https://doi.org/10.1038/35093011
Li S, Mecca A, Kim J, Caprara GA, Wagner EL, Du T-T, Petrov L, Xu W, Cui R, Rebustini IT, Kachar B, Peng AW, Shin J-B (2020) Myosin-VIIa is expressed in multiple isoforms and essential for tensioning the hair cell mechanotransduction complex. Nature Communications 11: 2066 https://doi.org/10.1038/s41467-020-15936-z
Schwander M, Lopes V, Sczaniecka A, Gibbs D, Lillo C, Delano D, Tarantino LM, Wiltshire T, Williams DS, Müller U (2009) A novel allele of myosin VIIa reveals a critical function for the C-Terminal FERM Domain for Melanosome Transport in Retinal Pigment Epithelial cells. J Neurosci 29:15810–15818. https://doi.org/10.1523/jneurosci.4876-09.2009
Calabro KR, Boye SL, Choudhury S, Fajardo D, Peterson JJ, Li W, Crosson SM, Kim M-J, Ding D, Salvi R, Someya S, Boye SE (2019) A Novel Mouse Model of MYO7A USH1B reveals auditory and visual system haploinsufficiencies. Front NeuroSci 13. https://doi.org/10.3389/fnins.2019.01255
Dougherty GW, Loges NT, Klinkenbusch JA, Olbrich H, Pennekamp P, Menchen T, Raidt J, Wallmeier J, Werner C, Westermann C, Ruckert C, Mirra V, Hjeij R, Memari Y, Durbin R, Kolb-Kokocinski A, Praveen K, Kashef MA, Kashef S, Eghtedari F, Häffner K, Valmari P, Baktai G, Aviram M, Bentur L, Amirav I, Davis EE, Katsanis N, Brueckner M, Shaposhnykov A, Pigino G, Dworniczak B, Omran H (2016) DNAH11 localization in the Proximal Region of Respiratory Cilia defines distinct outer dynein arm complexes. Am J Respir Cell Mol Biol 55:213–224. https://doi.org/10.1165/rcmb.2015-0353OC
Schultz R, Elenius V, Lukkarinen H, Saarela T (2020) Two novel mutations in the DNAH11 gene in primary ciliary dyskinesia (CILD7) with considerable variety in the clinical and beating cilia phenotype. BMC Med Genet 21:237. https://doi.org/10.1186/s12881-020-01171-2
Xiong Y, Xia H, Yuan L, Deng S, Ding Z, Deng H (2021) Identification of compound heterozygous DNAH11 variants in a Han-Chinese family with primary ciliary dyskinesia. J Cell Mol Med 25:9028–9037
Xia H, Huang X, Deng S, Xu H, Yang Y, Liu X, Yuan L, Deng H (2021) DNAH11 compound heterozygous variants cause heterotaxy and congenital heart disease. PLoS ONE 16:e0252786
Hörster F, Schwab MA, Sauer SW, Pietz J, Hoffmann GF, Okun JG, Kölker S, Kins S (2006) Phenylalanine reduces synaptic density in mixed cortical cultures from mice. Pediatr Res 59:544–548. https://doi.org/10.1203/01.pdr.0000203091.45988.8d
Thau-Zuchman O, Pallier PN, Savelkoul PJM, Kuipers AAM, Verkuyl JM, Michael-Titus AT (2022) High phenylalanine concentrations induce demyelination and microglial activation in mouse cerebellar organotypic slices. Front NeuroSci 16. https://doi.org/10.3389/fnins.2022.926023
Wegner AM, Nebhan CA, Hu L, Majumdar D, Meier KM, Weaver AM, Webb DJ (2008) N-wasp and the arp2/3 complex are critical regulators of actin in the development of dendritic spines and synapses. J Biol Chem 283:15912–15920. https://doi.org/10.1074/jbc.M801555200
Tao-Cheng J-H (2020) Stimulation-induced differential redistributions of clathrin and clathrin-coated vesicles in axons compared to soma/dendrites. Mol Brain 13:141. https://doi.org/10.1186/s13041-020-00683-5
Kumar V, Umair Z, Kumar S, Goutam RS, Park S, Kim J (2021) The regulatory roles of motile cilia in CSF circulation and hydrocephalus. Fluids and Barriers of the CNS 18:31. https://doi.org/10.1186/s12987-021-00265-0
De Luca C, Virtuoso A, Maggio N, Papa M (2017) Neuro-Coagulopathy: blood coagulation factors in Central Nervous System diseases. Int J Mol Sci 18. https://doi.org/10.3390/ijms18102128
Welch G, Tsai LH (2022) Mechanisms of DNA damage-mediated neurotoxicity in neurodegenerative disease. EMBO Rep 23:e54217. https://doi.org/10.15252/embr.202154217
Wang M, Zhou P, Zhang S, Liu X, Lv L, Wang Z, Ye F, Wang X, Jiang S (2018) Clinical features, treatment, and long-term outcomes of Central Neurocytoma: a 20-Year experience at a single Center. World Neurosurg 109:e59–e66. https://doi.org/10.1016/j.wneu.2017.09.103
Mahavadi AK, Patel PM, Kuchakulla M, Shah AH, Eichberg D, Luther EM, Komotar RJ, Ivan ME (2020) Central Neurocytoma Treatment modalities: a systematic review assessing the outcomes of combined maximal safe resection and Radiotherapy with Gross Total Resection. World Neurosurg 137:e176–e182. https://doi.org/10.1016/j.wneu.2020.01.114
Cao D, Chen Y, Guo Z, Ou Y, Chen J (2021) Clinical Outcome after Microsurgical Resection of Central Neurocytoma: a single-centre analysis of 15 years. Frontiers in Neurology 12
Han S, Yang Z, Yang Y, Qi X, Yan C, Yu C (2020) Individual treatment decisions for central neurocytoma. Front Neurol 11:834
Juratli TA, Geiger K, Leimert M, Schackert G, Kirsch M (2013) Atypical Central Neurocytoma with Recurrent Spinal Dissemination over a Period of 20 Years: A Case Report and Review of the Literature. Case Reports in Neurological Medicine 2013: 925647 https://doi.org/10.1155/2013/925647
Konovalov A, Maryashev S, Pitskhelauri D, Siomin V, Golanov A, Dalechina A (2021) The last decade’s experience of management of central neurocytomas: treatment strategies and new options. Surg Neurol Int 12:336–336. https://doi.org/10.25259/SNI_764_2020
Kim S, Wong YC, Gao F, Krainc D (2021) Dysregulation of mitochondria-lysosome contacts by GBA1 dysfunction in dopaminergic neuronal models of Parkinson’s disease. Nat Commun 12:1807. https://doi.org/10.1038/s41467-021-22113-3
Klein H-U, Trumpff C, Yang H-S, Lee AJ, Picard M, Bennett DA, De Jager PL (2021) Characterization of mitochondrial DNA quantity and quality in the human aged and Alzheimer’s disease brain. Mol Neurodegeneration 16:75. https://doi.org/10.1186/s13024-021-00495-8
Martín-Jiménez R, Lurette O, Hebert-Chatelain E (2020) Damage in mitochondrial DNA Associated with Parkinson’s Disease. DNA Cell Biol 39:1421–1430. https://doi.org/10.1089/dna.2020.5398
Uttara B, Singh AV, Zamboni P, Mahajan RT (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7:65–74. https://doi.org/10.2174/157015909787602823
Gegg ME, Schapira AHV (2016) Mitochondrial dysfunction associated with glucocerebrosidase deficiency. Neurobiol Dis 90:43–50. https://doi.org/10.1016/j.nbd.2015.09.006
Zheng W, Fan D (2022) Glucocerebrosidase Mutations Cause Mitochondrial and lysosomal dysfunction in Parkinson’s Disease: Pathogenesis and therapeutic implications. Front Aging Neurosci 14. https://doi.org/10.3389/fnagi.2022.851135
Dobrowolski SF, Phua YL, Sudano C, Spridik K, Zinn PO, Wang Y, Bharathi S, Vockley J, Goetzman E (2021) Phenylalanine hydroxylase deficient phenylketonuria comparative metabolomics identifies energy pathway disruption and oxidative stress. Mol Genet Metab. https://doi.org/10.1016/j.ymgme.2021.04.002
Zanon E, Pasca S (2019) Intracranial haemorrhage in children and adults with haemophilia A and B: a literature review of the last 20 years. Blood Transfus 17:378–384. https://doi.org/10.2450/2019.0253-18
Tschoe C, Bushnell CD, Duncan PW, Alexander-Miller MA, Wolfe SQ (2020) Neuroinflammation after Intracerebral Hemorrhage and potential therapeutic targets. J Stroke 22:29–46. https://doi.org/10.5853/jos.2019.02236
Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, Kaufman RJ (2008) Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci USA 105:18525–18530. https://doi.org/10.1073/pnas.0809677105
Guerra M, Sival D, Jimenez A, Dominguez Pinos MD, den Dunnen W, Bátiz LF, Pérez-Figares JM, Rodríguez E (2010) Defects in cell-cell junctions lead to neuroepithelial/ependymal denudation in the telencephalon of human hydrocephalic foetuses. Cerebrospinal Fluid Research 7:S56. https://doi.org/10.1186/1743-8454-7-S1-S56
Baumann N, Pham-dinh D (2002) Astrocytes. In: Ramachandran VS (ed) Encyclopedia of the human brain. Academic Press, New York, pp 251–268
Mirzadeh Z, Kusne Y, Duran-Moreno M, Cabrales E, Gil-Perotin S, Ortiz C, Chen B, Garcia-Verdugo JM, Sanai N, Alvarez-Buylla A (2017) Bi- and uniciliated ependymal cells define continuous floor-plate-derived tanycytic territories. Nat Commun 8:13759–13759. https://doi.org/10.1038/ncomms13759
Olstad EW, Ringers C, Hansen JN, Wens A, Brandt C, Wachten D, Yaksi E, Jurisch-Yaksi N (2019) Ciliary beating compartmentalizes Cerebrospinal Fluid Flow in the brain and regulates Ventricular Development. Curr Biol 29:229–241e226. https://doi.org/10.1016/j.cub.2018.11.059
Peng JM, Chen WY, Cheng JH, Luo JW, Tzeng HT (2021) Dysregulation of Cytoskeleton Remodeling drives invasive leading cells detachment. Cancers (Basel) 13. https://doi.org/10.3390/cancers13225648
Ren W, Zhao W, Cao L, Huang J (2021) Involvement of the Actin Machinery in programmed cell death. Front Cell Dev Biology 8. https://doi.org/10.3389/fcell.2020.634849
Egea G, Serra-Peinado C, Gavilan MP, Rios RM (2015) Cytoskeleton and golgi-apparatus interactions: a two-way road of function and structure
Koppers M, Özkan N, Farías GG (2020) Complex interactions between membrane-bound organelles, Biomolecular condensates and the Cytoskeleton. Front Cell Dev Biology 8. https://doi.org/10.3389/fcell.2020.618733
Smith CEL, Lake AVR, Johnson CA (2020) Primary cilia, Ciliogenesis and the actin cytoskeleton: a little less resorption, a little more actin please. Front cell Dev Biology 8:622822–622822. https://doi.org/10.3389/fcell.2020.622822
Hua K, Ferland RJ (2018) Primary cilia proteins: ciliary and extraciliary sites and functions. Cell Mol Life Sci 75:1521–1540. https://doi.org/10.1007/s00018-017-2740-5
Whittaker DE, Riegman KL, Kasah S, Mohan C, Yu T, Pijuan-Sala B, Hebaishi H, Caruso A, Marques AC, Michetti C, Smachetti ME, Shah A, Sabbioni M, Kulhanci O, Tee WW, Reinberg D, Scattoni ML, Volk H, McGonnell I, Wardle FC, Fernandes C, Basson MA (2017) The chromatin remodeling factor CHD7 controls cerebellar development by regulating reelin expression. J Clin Invest 127:874–887. https://doi.org/10.1172/jci83408
Ahmed M, Moon R, Prajapati RS, James E, Basson MA, Streit A (2021) The chromatin remodelling factor Chd7 protects auditory neurons and sensory hair cells from stress-induced degeneration. Commun Biol 4:1260–1260. https://doi.org/10.1038/s42003-021-02788-6
Kulanuwat S, Jungtrakoon P, Tangjittipokin W, Yenchitsomanus PT, Plengvidhya N (2018) Fanconi anemia complementation group C protection against oxidative stress–induced β–cell apoptosis. Mol Med Rep 18:2485–2491. https://doi.org/10.3892/mmr.2018.9163
Kalawi AZ, Malicki DM, Abdullaev Z, Pratt DW, Quezado M, Aldape K, Elster JD, Paul MR, Khanna PC, Levy ML, Crawford JR (2022) The role of methylation profiling in histologically diagnosed neurocytoma: a case series. J Neurooncol 159:725–733. https://doi.org/10.1007/s11060-022-04117-1
Uro-Coste E, Tauziede-Espariat A, Dubucs C, Chiforeanu DC, Siegfried A, Nicaise Y, Bauchet L, Riffaud L, Bielle F, Vasiljevic A, Appay R, Evrard S, Varlet P, Rigau V, The Biopathology R-LOCn (2023) Two novel tumours with NTRK2 fusion in the methylation class of extraventricular neurocytomas, including one intraventricular. Brain Pathol n/a e13223. https://doi.org/10.1111/bpa.13223
Sievers P, Stichel D, Schrimpf D, Sahm F, Koelsche C, Reuss DE, Wefers AK, Reinhardt A, Huang K, Ebrahimi A, Hou Y, Pajtler KW, Pfister SM, Hasselblatt M, Stummer W, Schick U, Hartmann C, Hagel C, Staszewski O, Reifenberger G, Beschorner R, Coras R, Keyvani K, Kohlhof P, Diomedi-Camassei F, Herold-Mende C, Giangaspero F, Rushing E, Giannini C, Korshunov A, Jones DTW, von Deimling A (2018) FGFR1:TACC1 fusion is a frequent event in molecularly defined extraventricular neurocytoma. Acta Neuropathol 136:293–302. https://doi.org/10.1007/s00401-018-1882-3
Zhang D, Yong W, Movassaghi M, Rodriguez FJ, Yang I, McKeever P, Qian J, Li JY, Mao Q, Newell KL, Green RM, Welsh CT, Heaney AP (2022) Whole exome sequencing identifies PHF14 mutations in Neurocytoma and predicts responsivity to the PDGFR inhibitor Sunitinib. Biomedicines 10. https://doi.org/10.3390/biomedicines10112842
Acknowledgements
We gratefully acknowledge Masoud Movassaghi (University of Southern California), Fausto J Rodriguez and Issac Yang (UCLA David Geffen School of Medicine), Paul McKeever (University of Michigan), Jiang Qian (Albany Medical Center), Jian Yi Li (North Shore University Hospital and Long Island Jewish Medical Center, Northwell Health System), Qinwen Mao (University of Utah), Kathy L Newell (Indiana University School of Medicine), Cynthia T Welsh (Medical University of South Carolina) for contributing neurocytoma tissues.
Funding
We are grateful to the Warley Trust for financial support for these studies.
Author information
Authors and Affiliations
Contributions
SK: data analysis and wrote first manuscript draft; DZ: sample collection, processing and whole exome sequencing; WY: histopathological investigation of the clinical samples; APH: Conceived the study, interpreted data and assisted manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khowal, S., Zhang, D., Yong, W.H. et al. Whole-exome sequencing reveals genetic variants that may play a role in neurocytomas. J Neurooncol 166, 471–483 (2024). https://doi.org/10.1007/s11060-024-04567-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-024-04567-9