Abstract
Purpose
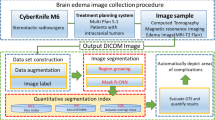
This paper presents a deep learning model for use in the automated segmentation of metastatic brain tumors and associated perilesional edema.
Methods
The model was trained using Gamma Knife surgical data (90 MRI sets from 46 patients), including the initial treatment plan and follow-up images (T1-weighted contrast-enhanced (T1cWI) and T2-weighted images (T2WI)) manually annotated by neurosurgeons to indicate the target tumor and edema regions. A mask region-based convolutional neural network was used to extract brain parenchyma, after which the DeepMedic 3D convolutional neural network was in the segmentation of tumors and edemas.
Results
Five-fold cross-validation demonstrated the efficacy of the brain parenchyma extraction model, achieving a Dice similarity coefficient of 96.4%. The segmentation models used for metastatic tumors and brain edema achieved Dice similarity coefficients of 71.6% and 85.1%, respectively. This study also presents an intuitive graphical user interface to facilitate the use of these models in clinical analysis.
Conclusion
This paper introduces a deep learning model for the automated segmentation and quantification of brain metastatic tumors and perilesional edema trained using only T1cWI and T2WI. This technique could facilitate further research on metastatic tumors and perilesional edema as well as other intracranial lesions.


Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are not publicly available, but they are available from the corresponding author on reasonable request. Note that all data was anonymized during the data evaluation process.
References
Fox BD, Cheung VJ, Patel AJ, Suki D, Rao G (2011) Epidemiology of metastatic brain tumors. Neurosurg Clin N Am 22:1–6. https://doi.org/10.1016/j.nec.2010.08.007
Soffietti R, Rudā R, Mutani R (2002) Management of brain metastases. J Neurol 249:1357–1369. https://doi.org/10.1007/s00415-002-0870-6
Senger DR, Van de Water L, Brown LF, Nagy JA, Yeo KT, Yeo TK, Berse B, Jackman RW, Dvorak AM, Dvorak HF (1993) Vascular permeability factor (VPF, VEGF) in Tumor biology. Cancer Metastasis Rev 12:303–324. https://doi.org/10.1007/bf00665960
Wick W, Küker W (2004) Brain edema in neurooncology: radiological assessment and management. Onkologie 27:261–266. https://doi.org/10.1159/000077976
Tran TT, Jilaveanu LB, Omuro A, Chiang VL, Huttner A, Kluger HM (2019) Complications associated with immunotherapy for brain metastases. Curr Opin Neurol 32:907–916. https://doi.org/10.1097/wco.0000000000000756
Jha RM, Raikwar SP, Mihaljevic S, Casabella AM, Catapano JS, Rani A, Desai S, Gerzanich V, Simard JM (2021) Emerging therapeutic targets for cerebral edema. Expert Opin Ther Targets 25:917–938. https://doi.org/10.1080/14728222.2021.2010045
Chou CJ, Lee CC, Chen CJ, Yang HC, Peng SJ (2021) Displacement of Gray Matter and Incidence of Seizures in patients with cerebral cavernous malformations. Biomedicines 9 https://doi.org/10.3390/biomedicines9121872
He K, Gkioxari G, Dollar P, Girshick R (2020) Mask R-CNN. IEEE Trans Pattern Anal Mach Intell 42:386–397. https://doi.org/10.1109/tpami.2018.2844175
Kamnitsas K, Ledig C, Newcombe VFJ, Simpson JP, Kane AD, Menon DK, Rueckert D, Glocker B (2017) Efficient multi-scale 3D CNN with fully connected CRF for accurate brain lesion segmentation. Med Image Anal 36:61–78. https://doi.org/10.1016/j.media.2016.10.004
Saha BN, Ray N, Greiner R, Murtha A, Zhang H (2012) Quick detection of brain tumors and edemas: a bounding box method using symmetry. Comput Med Imaging Graph 36:95–107. https://doi.org/10.1016/j.compmedimag.2011.06.001
Njeh I, Sallemi L, Ayed IB, Chtourou K, Lehericy S, Galanaud D, Hamida AB (2015) 3D multimodal MRI brain glioma Tumor and edema segmentation: a graph cut distribution matching approach. Comput Med Imaging Graph 40:108–119. https://doi.org/10.1016/j.compmedimag.2014.10.009
Zhang C, Shen X, Cheng H, Qian Q (2019) Brain Tumor Segmentation Based on Hybrid Clustering and Morphological Operations. Int J Biomed Imaging 2019: 7305832. https://doi.org/10.1155/2019/7305832
Demirhan A, Toru M, Guler I (2015) Segmentation of Tumor and edema along with healthy tissues of brain using wavelets and neural networks. IEEE J Biomed Health Inform 19:1451–1458. https://doi.org/10.1109/jbhi.2014.2360515
Pennig L, Shahzad R, Caldeira L, Lennartz S, Thiele F, Goertz L, Zopfs D, Meißner AK, Fürtjes G, Perkuhn M, Kabbasch C, Grau S, Borggrefe J, Laukamp KR (2021) Automated detection and segmentation of Brain metastases in Malignant Melanoma: evaluation of a dedicated Deep Learning Model. AJNR Am J Neuroradiol 42:655–662. https://doi.org/10.3174/ajnr.A6982
Garde E, Mortensen EL, Krabbe K, Rostrup E, Larsson HB (2000) Relation between age-related decline in intelligence and cerebral white-matter hyperintensities in healthy octogenarians: a longitudinal study. Lancet 356:628–634. https://doi.org/10.1016/s0140-6736(00)02604-0
Haller S, Kövari E, Herrmann FR, Cuvinciuc V, Tomm AM, Zulian GB, Lovblad KO, Giannakopoulos P, Bouras C (2013) Do brain T2/FLAIR white matter hyperintensities correspond to myelin loss in normal aging? A radiologic-neuropathologic correlation study. Acta Neuropathol Commun 1:14. https://doi.org/10.1186/2051-5960-1-14
Kerschbaumer J, Bauer M, Popovscaia M, Grams AE, Thomé C, Freyschlag CF (2017) Correlation of Tumor and Peritumoral Edema Volumes with survival in patients with cerebral metastases. Anticancer Res 37:871–875. https://doi.org/10.21873/anticanres.11392
Pan HC, Sun MH, Chen CC, Chen CJ, Lee CH, Sheehan J (2008) Neuroimaging and quality-of-life outcomes in patients with brain Metastasis and peritumoral edema who undergo Gamma Knife Surgery. J Neurosurg 109 Suppl:90–98. https://doi.org/10.3171/jns/2008/109/12/s15
Fábián K, Gyulai M, Furák J, Várallyay P, Jäckel M, Bogos K, Döme B, Pápay J, Tímár J, Szállási Z, Moldvay J (2016) Significance of primary Tumor Location and Histology for Brain Metastasis Development and Peritumoral Brain Edema in Lung Cancer. Oncology 91:237–242. https://doi.org/10.1159/000447517
Yaltirik Bilgin E, Unal O, Ciledag N (2022) Vasogenic Edema Pattern in Brain Metastasis. J Coll Physicians Surg Pak 32:1020–1025. https://doi.org/10.29271/jcpsp.2022.08.1020
Tini P, Nardone V, Pastina P, Battaglia G, Vinciguerra C, Carfagno T, Rubino G, Carbone SF, Sebaste L, Cerase A, Federico A, Pirtoli L (2017) Perilesional edema in brain Metastasis from non-small cell Lung cancer (NSCLC) as predictor of response to radiosurgery (SRS). Neurol Sci 38:975–982. https://doi.org/10.1007/s10072-017-2876-y
Meyer HJ, Hamerla G, Höhn AK, Hoffmann KT, Surov A (2021) Perifocal edema volume is not associated with immunohistochemical features reflecting proliferation potential, microvessel density, neoangiogenesis and invasiveness in brain Metastasis. Clin Neurol Neurosurg 202:106537. https://doi.org/10.1016/j.clineuro.2021.106537
Funding
This work was financially supported in part by the National Science and Technology Council, Taiwan, under the project NSTC 112-2628-E-038-001-MY3, and in part by the Higher Education Sprout Project by the Ministry of Education (MOE), Taiwan. We thank the National Center for High-performance Computing (NCHC) of National Applied Research Laboratories (NARLabs) in Taiwan for providing computational and storage resources.
Author information
Authors and Affiliations
Contributions
Conceptualization, supervision, and project administration were performed by Chi-Jen Chou and Syu-Jyun Peng. The original draft was written by Chi-Jen Chou and Syu-Jyun Peng and all authors commented on previous versions of the manuscript. Data curation, formal analysis, software and methodology selection were performed by Chi-Jen Chou, Huai-Che Yang, Po-Yao Chang, and Syu-Jyun Peng. Investigation, data sourcing, and visualization were performed by Chi-Jen Chou, Huai-Che Yang, Ching-Jen Chen, Hsiu-Mei Wu, Chun-Fu Lin, I-Chun Lai, and Syu-Jyun Peng. All authorshave read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chou, CJ., Yang, HC., Chang, PY. et al. Automated identification and quantification of metastatic brain tumors and perilesional edema based on a deep learning neural network. J Neurooncol 166, 167–174 (2024). https://doi.org/10.1007/s11060-023-04540-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04540-y