Abstract
Introduction
Glioblastoma multiforme (GBM) is the most common primary intracranial malignancy; survival can be improved by maximizing the extent-of-resection.
Methods
A near-infrared fluorophore (Indocyanine-Green, ICG) was combined with a photosensitizer (Chlorin-e6, Ce6) on the surface of superparamagnetic-iron-oxide-nanoparticles (SPIONs), all FDA-approved for clinical use, yielding a nanocluster (ICS) using a microemulsion. The physical–chemical properties of the ICS were systematically evaluated. Efficacy of photodynamic therapy (PDT) was evaluated in vitro with GL261 cells and in vivo in a subtotal resection trial using a syngeneic flank tumor model. NIR imaging properties of ICS were evaluated in both a flank and an intracranial GBM model.
Results
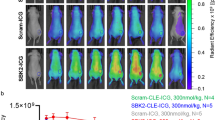
ICS demonstrated high ICG and Ce6 encapsulation efficiency, high payload capacity, and chemical stability in physiologic conditions. In vitro cell studies demonstrated significant PDT-induced cytotoxicity using ICS. Preclinical animal studies demonstrated that the nanoclusters can be detected through NIR imaging in both flank and intracranial GBM tumors (ex: 745 nm, em: 800 nm; mean signal-to-background 8.5 ± 0.6). In the flank residual tumor PDT trial, subjects treated with PDT demonstrated significantly enhanced local control of recurrent neoplasm starting on postoperative day 8 (23.1 mm3 vs 150.5 mm3, p = 0.045), and the treatment effect amplified to final mean volumes of 220.4 mm3 vs 806.1 mm3 on day 23 (p = 0.0055).
Conclusion
A multimodal theragnostic agent comprised solely of FDA-approved components was developed to couple optical imaging and PDT. The findings demonstrated evidence for the potential theragnostic benefit of ICS in surgical oncology that is conducive to clinical integration.





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Tamimi AF, Juweid M (2017) Epidemiology and outcome of glioblastoma. In: Glioblastoma; Exon Publication, Brisbane. p 143
Johnson DR, O’Neill BP (2012) Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol 107:359
Wallner KE, Galicich JH, Krol G et al (1989) Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys 16:1405
Sanai N, Polley M-Y, McDermott MW et al (2011) An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115:3–8
Lacroix M, Abi-Said D, Fourney DR et al (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95:190–198
Brown TJ, Brennan MC, Li M et al (2016) Association of the extent of resection with survival in glioblastoma. JAMA Oncol 2:1460
Orringer D, Lau D, Khatri S et al (2012) Extent of resection in patients with glioblastoma: limiting factors, perception of resectability, and effect on survival. J Neurosurg 117:851
Stummer W, Pichlmeier U, Meinel T et al (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma : a randomised controlled multicentre phase III trial. Lancet Oncol 7:392–401
Hong G, Antaris AL, Dai H (2017) Near-infrared fluorophores for biomedical imaging. Nat.Biomed. Eng 1:1–22
Schols RM, Connell NJ, Stassen LPS (2015) Near-infrared fluorescence imaging for real-time intraoperative anatomical guidance in minimally invasive surgery: a systematic review of the literature. World J Surg 39:1069–1079
Lee JYK, Thawani JP, Pierce J et al (2016) Intraoperative near-infrared optical imaging can localize gadolinium-enhancing gliomas during surgery. Neurosurgery 79:856–871
Lee JYK, Pierce JT, Zeh R et al (2017) Intraoperative near-infrared optical contrast can localize brain metastases. World Neurosurg 106:120–130
Lee JYK, Pierce JT, Thawani JP et al (2017) Near-infrared fluorescent image-guided surgery for intracranial meningioma. Neurosurgery 128:380–390
Carr JA, Franke D, Caram JR et al (2018) Shortwave infrared fluorescence imaging with the clinically approved near-infrared dye indocyanine green. Proc Natl Acad Sci USA 115:4465
Maruyama T, Muragaki Y, Nitta M et al (2016) Photodynamic therapy for malignant brain tumors. Japanese J Neurosurg 25:895
Eljamel S (2010) Photodynamic applications in brain tumors: a comprehensive review of the literature. Photodiagnosis Photodyn Ther 7:76
Eljamel MS, Goodman C, Moseley H (2008) ALA and Photofrin® Fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: a single centre Phase III randomised controlled trial. Lasers Med Sci 23:361
Li Y, Yu Y, Kang L, Lu Y (2014) Effects of chlorin e6-mediated photodynamic therapy on human colon cancer SW480 cells. Int J Clin Exp Med 7:4867
Nann T (2011) Nanoparticles in photodynamic therapy. Nano Biomed Eng 3:137
Paul S, Heng PWS, Chan LW (2013) Optimization in solvent selection for chlorin e6 in photodynamic therapy. J Fluoresc 23:283
Kelkar SS, Reineke TM (2011) Theranostics: combining imaging and therapy. Bioconjug Chem 22:1879–1903
Lee JH, Lee K, Moon SH et al (2009) All-in-One target-cell-specific magnetic nanoparticles for simultaneous molecular imaging and siRNA delivery. Angew Chem Int Ed Engl 48:4174–4179
Thawani JP, Amirshaghaghi A, Yan L et al (2017) Photoacoustic-guided surgery with indocyanine green-coated superparamagnetic iron oxide nanoparticle clusters. Small 13:1–9
Kircher MF, De La Zerda A, Jokerst JV et al (2012) A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat Med 18:829–834
Li J, Zhao J, Tan T et al (2020) Nanoparticle drug delivery system for glioma and its efficacy improvement strategies: a comprehensive review. Int J Nanomed 15:2563–2582
Guo J, Gao X, Su L et al (2011) Aptamer-functionalized PEG-PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials 32:8010–8020
Jordan A, Scholz R, Maier-Hauff K et al (2006) The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. J Neurooncol 78:7–14
Michael JS, Lee B-S, Zhang M, Yu JS (2018) Nanotechnology for treatment of glioblastoma multiforme. J Transl Intern Med 6:128–133
Pardridge WM (2005) The blood-brain barrier and neurotherapeutics. NeuroRx 2:1–2
Chen H, Zhen Z, Todd T et al (2013) Nanoparticles for improving cancer diagnosis. Mater Sci Eng R Reports 74:35–69
Chenthamara D, Subramaniam S, Ramakrishnan SG et al (2019) Therapeutic efficacy of nanoparticles and routes of administration. Biomater Res 23:20
Huynh E, Zheng G (2013) Engineering multifunctional nanoparticles: all-in-one versus one-for-all. Wiley Interdiscip Rev Nanomed Nanobiotechnol 5:250–265
Higbee-Dempsey E, Amirshaghaghi A, Case MJ et al (2019) Indocyanine green–coated gold nanoclusters for photoacoustic imaging and photothermal therapy. Adv Ther 2:1900088
Amirshaghaghi A, Yan L, Miller J et al (2019) Chlorin e6-coated superparamagnetic iron oxide nanoparticle (SPION) nanoclusters as a theranostic agent for dual-mode imaging and photodynamic therapy. Sci Rep 9:1–9
Yan L, Amirshaghaghi A, Huang D et al (2018) Protoporphyrin IX (PpIX)-coated superparamagnetic iron oxide nanoparticle (SPION) nanoclusters for magnetic resonance imaging and photodynamic therapy. Adv Funct Mater 28:1707030
Munnier E, Cohen-Jonathan S, Linassier C et al (2008) Novel method of doxorubicin–SPION reversible association for magnetic drug targeting. Int J Pharm 363:170–176
Husain SR, Behari N, Kreitman RJ et al (1998) Complete regression of established human glioblastoma tumor xenograft by interleukin-4 toxin therapy. Cancer Res 58:3649–3653
Zeh R, Sheikh S, Xia L et al (2017) The second window ICG technique demonstrates a broad plateau period for near infrared fluorescence tumor contrast in glioblastoma. PLoS ONE 12:e0182034
Baumann BC, Dorsey JF, Benci JL et al (2012) Stereotactic intracranial implantation and in vivo bioluminescent imaging of tumor xenografts in a mouse model system of glioblastoma multiforme. J Vis Exp 67:e4089
Yuan A, Tang X, Qiu X et al (2015) Activatable photodynamic destruction of cancer cells by NIR dye/photosensitizer loaded liposomes. Chem Commun 51:3340–3342
Herve P, Nome F, Fendler JH (1984) Magnetic effects on chemical reactions in the absence of magnets. Effects of surfactant vesicle entrapped magnetite particles on benzophenone photochemistry. J Am Chem Soc 106:8291.
Saravanakumar G, Kim J, Kim WJ (2016) Reactive-oxygen-species-responsive drug delivery systems: promises and challenges. Adv Sci 4:1600124
Enochs WS, Harsh G, Hochberg F, Weissleder R (1999) Improved delineation of human brain tumors on MR images using a long- circulating, superparamagnetic iron oxide agent. J Magn Reson Imaging 9:228
Madajewski B, Judy BF, Mouchli A et al (2012) Intraoperative near-infrared imaging of surgical wounds after tumor resections can detect residual disease. Clin Cancer Res 18:5741–5752
Raucher D, Dragojevic S, Ryu J (2018) Macromolecular drug carriers for targeted glioblastoma therapy: preclinical studies, challenges, and future perspectives. Front Oncol 8:624
Béduneau A, Saulnier P, Benoit J-P (2007) Active targeting of brain tumors using nanocarriers. Biomaterials 28:4947–4967
Xin H, Jiang X, Gu J et al (2011) Angiopep-conjugated poly(ethylene glycol)-co-poly(ε-caprolactone) nanoparticles as dual-targeting drug delivery system for brain glioma. Biomaterials 32:4293–4305
Jackson H, Muhammad O, Daneshvar H, et al (2007) Quantum dots are phagocytized by macrophages and colocalize with experimental gliomas. Neurosurgery 60:524–530
Rosenblum D, Joshi N, Tao W et al (2018) Progress and challenges towards targeted delivery of cancer therapeutics. Nat Commun 9:1–12
Amirshaghaghi A, Altun B, Nwe K et al (2018) Site-specific labeling of cyanine and porphyrin dye-stabilized nanoemulsions with affibodies for cellular targeting. J Am Chem Soc 140:13550–13553
Funding
National Institutes of Health (R01EB029238 to A.T., R21 EB023989 to A.T., R01NS100892 to Z.C., R01CA175480 to Z.C., P01CA087971 to T.M.B., P30CA016520 to Z.C., T.M.B., and A.T., R01CA236362 to T.M.B.); National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR000003 to JYKL, TL1TR001880 to SS Cho); The U. of Penn. University Research Foundation Award to A.T.; Institute for Translational Medicine and Therapeutics of the Perelman School of Medicine at the University of Pennsylvania to JYKL;
Author information
Authors and Affiliations
Contributions
CWT and AA contributed equally to this work. CWT, AA, AT and JYKL designed the research; CWT, AA, SS Cho, SS Cai, EDR, YS performed the research; JM, S. Sheikh, ED, ZC, TMB, JFD, S Singhal supplied critical research resource and space; CWT, AA and SS Cho analyzed data; CWT and AA wrote the manuscript. All authors edited and reviewed the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All animal procedus were conducted according to a protocol approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania (#805979, #806516) and NIH and ARRIVE guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Teng, C.W., Amirshaghaghi, A., Cho, S.S. et al. Combined fluorescence-guided surgery and photodynamic therapy for glioblastoma multiforme using cyanine and chlorin nanocluster. J Neurooncol 149, 243–252 (2020). https://doi.org/10.1007/s11060-020-03618-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03618-1