Abstract
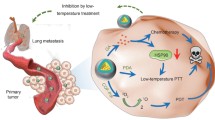
Photothermal therapy (PTT) can kill the tumor in situ, but fail to trigger strong antitumor immune response against its recurrence and metastasis. Herein, mannose-grafting Fe-doped carbon dots (M/Fe-CDs) were prepared via hydrothermal carbonization and chemical modification. The prepared M/Fe-CDs had characteristics of excellent optical qualities, good water solubility, and outstanding biocompatibility. Compared with pure CDs, the doping of Fe atom endowed the M/Fe-CDs with a high photothermal conversion efficiency (from 12.1 up to 30.1%). Under 808-nm NIR irradiation, the M/Fe-CDs could lead to local hyperthermia to kill tumor cells. Importantly, the M/Fe-CDs were capable of loading CpG-ODNs via electrostatic absorption and delivering them into the cytoplasm of dendritic cells (DCs). Finally, the CpG-ODNs-loading M/Fe-CDs could significantly increase the CD80/86 expression on the DCs surface. These results demonstrated that the M/Fe-CDs could be used as a versatile nanoplatform to combine the PTT and immune activation for tumor treatment.








Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Yu Y, Tang D, Liu C (2022) Biodegradable polymer with effective NIR-II absoption as a photothermal agent for deep tumor therapy. Adv Mater 34(4):2105976
Liu J, Liang H, Li M, Luo Z, Zhang J et al (2018) Tumor acidity activating multifunctional nanoplatform for NIR-mediated multiple enhanced photodynamic and photothermal tumor therapy. Biomaterials 157:107–124
Huang X, Lu Y, Guo M, Du S, Han N (2021) Recent strategies for nano-based PTT combined with immunotherapy: from a biomaterial point of view. Theranostics 11(15):7546–7569
Abadeer N, Murphy C (2016) Recent progress in cancer thermal therapy using gold nanoparticles. J Phys Chem 2016:1171–1176
Kausar A (2019) Polymer/carbon-based quantum dot nanocomposite: forthcoming materials for technical application. J Macromol Sci 56(4):341–356
Xu A, Wang G, Li Y, Dong H, Yang S, He P, Ding G (2020) Carbon-based quantum dots with solid-state photoluminescent: mechanism, implementation, and application. Small 16(48):2004621
Su Y, Liu S, Guan Y, Xie Z, Jin X (2020) Renal clearable Hafnium-doped carbon dots for CT/Fluorescence imaging of orthotopic liver cancer. Biomaterials 255:120110
Li X, Vinothini K, Ramesh T, Rajan M, Ramu A (2020) Combined photodynamic-chemotherapy investigation of cancer cells using carbon quantum dot-based drug carrier system. Drug Delivery 27(1):791–804
Jin L, Shao Y, Chen X (2022) Dual-excitation red-emissive carbon dots excited by ultraviolet light for the mitochondria-targetable imaging and monitoring of biological process in living cells. J Photochem Photobiol A Chem 425:113702
Liu C, Zhang P, Zhai X, Tian F, Li W et al (2012) Nano-carrier for gene delivery and bioimaging based on carbon dots with PEI-passivation enhanced fluorescence. Biomaterials 33(13):3604–3613
Wang B, Song H, Qu X, Chang J, Liu S (2021) Carbon dots as a new class of nanomedicines: opportunities and challenges. Coord Chem Rev 442:214010
Ge J, Jia Q, Liu W, Guo L, Liu Q et al (2015) Red-emissive carbon dots for fluorescent, photoacoustic, and thermal theranostics in living mice. Adv Mater 27(28):4169–4177
Sweeney E, Cano-Mejia J, Fernandes R (2018) Photothermal therapy generates a thermal window of immunogenic cell death in neuroblastoma. Small 2018:1800678
Li L, Zou J, Dai Y, Fan W, Chen X (2020) Burst release of encapsulated annexin A5 in tumours boosts cytotoxic T-cell responses by blocking the phagocytosis of apoptotic cells. Nat Biomed Eng 2020:1–15
Fan M, Le J, Pang M, Yang X, Yang Y et al (2021) Injectable adhesive hydrogel as photothermal-derived antigen reservoir for enhanced anti-tumor immunity. Adv Func Mater 31(20):1–16. https://doi.org/10.1002/adfm.202010587
An H, Cheng, Qian X, Cao (2010) Regulation of Toll-like receptor signaling in innate immunity. Sci China 53(001):34–43
Fitzgerald K, Kagan J (2020) Toll-like receptors and the control of immunity. Cell 180(6):1044–1066
Jia X, Guo J, Guo S, Zhao T, Luo E (2021) Antitumor effects and mechanisms of CpG ODN combined with attenuated Salmonella-delivered siRNAs against PD-1. Int Immunopharmacol 2021(90):90
Nor M, Steeneveld W, Mourits M, Hogeveen H (2015) The optimal number of heifer calves to be reared as dairy replacements. J Dairy Sci 98(2):861–871
Tao C, Zhu Y, Li X, Hanagata N (2015) Binding of CpG oligodeoxynucleotides to mesoporous silica nanoparticles for enhancing delivery efficiency. Microporous Mesoporous Mater 204:91–98
Han Q, Wang X, Jia X, Cai S, Liang W et al (2017) CpG loaded MoS2 nanosheets as multifunctional agents for photothermal enhanced cancer immunotherapy. Nanoscale 9(18):5927–5934
Zhang H, Gao X (2017) Nanodelivery systems for enhancing the immunostimulatory effect of CpG oligodeoxynucleotides. Mater Sci Eng 70(2):935–946
Jiang M, Zhang J, Shi Y, Yang W, Zheng N (2020) A trustworthy CpG nanoplatform for highly safe and efficient cancer photothermal combined immunotherapy. Nanoscale 12(6)
Wang Y, Xiang Y, Xin W, Wang X, Xin H (2020) Dendritic cell biology and its role in tumor immunotherapy. Hematol Oncol 13(1):107
Wang X, Wang Y, Xue Z (2023) Magnetic liposome as a dual-targeting delivery system for idiopathic pulmonary fibrosis treatment. J Colloid Interface Sci 636:388–400
Liu Z, Wang X, Chen X (2023) Construction of pH-responsive polydopamine coated magnetic layered hydroxide nanostructure for intracellular drug delivery. Eur J Pharm Biopharm 182:12–20
Cui L, Wang X, Liu Z (2023) Metal-organic framework decorated with glycyrrhetinic acid conjugated chitosan as a pH-responsive nanocarrier for targeted drug delivery. Int J Biol Macromol 240:124370
O’Neal D, Hirschb L, Halas N, Payne J, West J (2004) Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett 209(2):171–176
Funding
This work was financially supported by the Zhenjiang Key Research and Development Program-Social Development (SH2021030, SH2022100), the Young Talent Development Plan of Changzhou Health Commission (CZQM 2022018), and the Baoshan District Medical Health Project (20-E-11).
Author information
Authors and Affiliations
Contributions
Haiyang Ye, Xiaonan Qiu, Li Zhang, and Jianghong Qiu are responsive for experimental operation. Yunchao Wu, Wenqing Xu, and Xiao Xu are responsive for data collection and analysis. Huijun Yu, Shengqiang Zou, Guojun Zheng are responsive for data processing and drawing. Fengyi Du is responsive for experimental design and writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ye, H., Zhang, L., Qiu, X. et al. Construction of mannose-grafting Fe-doped carbon dots for CpG delivery and photothermal ablation of tumor. J Nanopart Res 26, 25 (2024). https://doi.org/10.1007/s11051-024-05936-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-024-05936-1