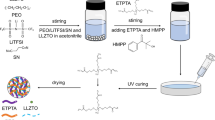
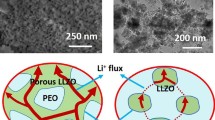
Abstract
Composite polymer electrolyte (CPE) with ceramic fillers has gained great attention for lithium batteries with high energy density and safety. However, the agglomeration of ceramic fillers and weak polymer-ceramic interaction induces limited ionic conductivity and hinders its implementation. Here, hollow multishelled structure (HoMS) ZnO with a size range of 700 ~ 900 nm is designed as fillers for polyethylene oxide (PEO)-based CPE. Strong chemical and mechanical interaction between PEO and ZnO HoMS enable a high ionic conductivity and good electrochemical and mechanical stability. Wherein, double-shelled ZnO HoMS exhibits a good ionic conductivity of 1.04 × 10−4 S∙cm−1 and 1.2 × 10−3 S∙cm−1 at 30 ℃ and 60 ℃. Additionally, all-solid-state LiFePO4/Li full cell adopted with ZnO HoMS filled CPE exhibits a high initial specific capacity of 169 mAh∙g−1 and good cycling stability and withstands abuse test. The enhanced performance is due to that HoMS provides PEO with faster ion transport channels, more effective Lewis acid-based interaction sites, suppressed PEO crystallinity, and improved ionic conductivity.





Similar content being viewed by others
Data availability
The original data are available from corresponding authors upon reasonable request.
References
Fan LZ, He H, Nan CW (2021) Tailoring inorganic-polymer composites for the mass production of solid-state batteries. Nat Rev Mater 6:1003–1019. https://doi.org/10.1038/s41578-021-00320-0
Li BQ, Peng HJ, CX et al (2019) Polysulfide electrocatalysis on framework porphyrin in high-capacity and high-stable lithium-sulfur batteries. CCS Chem 1: 128–137. https://doi.org/10.31635/ccschem.019.20180016
Rameez R, Nana Z, Ying X et al (2020) Electrocatalytic conversion of lithium polysulfides by highly dispersed ultrafine Mo2C nanoparticles on hollow N-doped carbon flowers for Li-S batteries. EcoMat 2:e12020. https://doi.org/10.1002/eom2.12020
Dias FB, Plomp L, Veldhuis JBJ (2000) Trends in polymer electrolytes for secondary lithium batteries. J Power Sources 88:169–191. https://doi.org/10.1016/S0378-7753(99)00529-7
Guo Y, Wu SC, He YB et al (2022) Solid-state lithium batteries: safety and prospects. eScience 2: 138–163. https://www.sciencedirect.com/science/article/pii/S2667141722000209?via%3Dihub. Accessed 2 Jan 2023
Hong Y, Jia L, Yang L et al (2020) Toward practical all-solid-state batteries with sulfide electrolyte: a review. Chem Res Chinese Universities 36:377–385. https://doi.org/10.1007/s40242-020-0103-5
Kairui G, Shao QL, Gong C et al (2022) One-pot synthesis of polyester-based linear and graft copolymers for solid polymer electrolytes. CCS Chem 4:3134–3149. https://doi.org/10.31635/ccschem.021.202101364
Li L, De CZ, Xi JX et al (2021) Challenges and development of composite solid electrolytes for all-solid-state lithium batteries. Chem Res Chinese Universities 37:210–231. https://doi.org/10.1007/s40242-021-0007-z
Xu SJ, Sun ZH, Sun CG (2020) Homogeneous and fast ion conduction of PEO-based solid-state electrolyte at low temperature. Adv Funct Mater 30:2007172. https://doi.org/10.1002/adfm.202007172
Zhang XD, Yue FS, Liang JY et al (2020) Structure design of cathode electrodes for solid-state batteries: challenges and progress. Small Struct 1:2000042. https://doi.org/10.1002/sstr.202000042
Croce F, Appetecchi GB, Persi L et al (1998) Nanocomposite polymer electrolytes for lithium batteries. Nature 394:456–458. https://doi.org/10.1038/28818
Jia Z, Yuan W, Zhao H et al (2014) Composite electrolytes comprised of poly (ethylene oxide) and silica nanoparticles with grafted poly (ethylene oxide)-containing polymers. RSC Adv 4:41087–41098. https://doi.org/10.1039/C4RA07262F
Wan J, Xie J, Kong X et al (2019) Ultrathin, flexible, solid polymer composite electrolyte enabled with aligned nanoporous host for lithium batteries. Nat Nanotechnol 14:705–711. https://doi.org/10.1038/s41565-019-0465-3
Xiong HM, Zhao X, Chen JS (2001) New polymer-inorganic nanocomposites: PEO-ZnO and PEO-ZnO-LiClO4 films. J Phys Chem B 105:10169–10174. https://doi.org/10.1021/jp0103169
Ding WQ, Lv F, Xu N et al (2021) Polyethylene oxide-based solid-state composite polymer electrolytes for rechargeable lithium batteries. ACS Appl Energy Mater 4:4581–4601. https://doi.org/10.1021/acsaem.1c00216
Li B, Bi R, Yang M et al (2022) Coating conductive polypyrrole layers on multiple shells of hierarchical SnO2 spheres and their enhanced cycling stability as lithium-ion battery anode. Appl Surf Sci 586:152836. https://doi.org/10.1016/j.apsusc.2022.152836
Li B, Wang J, Bi RY et al (2022) Accurately localizing multiple nanoparticles in a multishelled matrix through shell-to-core evolution for maximizing energy-storage capability. Adv Mater 34:2200206. https://doi.org/10.1002/adma.202200206
Wang J, Cui Y, Wang D (2020) Hollow multishelled structures revive high energy density batteries. Nanoscale Horiz 5:1287–1292. https://doi.org/10.1039/D0NH00311E
Dong Z, Lai X, Halpert JE et al (2012) Accurate control of multishelled ZnO hollow microspheres for dye-sensitized solar cells with high efficiency. Adv Mater 24:1046–1049. https://doi.org/10.1002/adma.201104626
Wang J, Wang Z, Mao D et al (2022) The development of hollow multishelled structure: from the innovation of synthetic method to the discovery of new characteristics. Sci China Chem 65:7–19. https://doi.org/10.1007/s11426-021-1097-9
Wang J, Yang M, Wang D (2022) Progress and perspectives of hollow multishelled structures. Chinese J Chem 40:1190–1203. https://doi.org/10.1002/cjoc.202100932
Wang L, Wan J, Wang J et al (2020) Small structures bring big things: performance control of hollow multishelled structures. Small Struct 2:2000041. https://doi.org/10.1002/sstr.202000041
Yang M, Bi R, Wang J et al (2022) Decoding lithium batteries through advanced in situ characterization techniques. Int J Min Met Mater 29:965–989. https://doi.org/10.1007/s12613-022-2461-0
Zhang X, He Y, Wei Y et al (2021) Carving the shell thickness of tungsten trioxide hollow multi-shelled structures for enhanced photocatalytic performance. Mater Chem Front 5:8010–8017. https://doi.org/10.1039/D1QM01124C
Zhao D, Yang N, Wei Y et al (2020) Sequential drug release via chemical diffusion and physical barriers enabled by hollow multishelled structures. Nature Commun 11:1–7. https://doi.org/10.1038/s41467-020-18177-2
Wang J, Tang H, Zhang L et al (2016) Multi-shelled metal oxides prepared via an anion-adsorption mechanism for lithium-ion batteries. Nat Energy 1:16050. https://doi.org/10.1038/nenergy.2016.50
Cong W, Jiang YW, Wen PH et al (2020) Controllable synthesis of hollow multishell structured Co3O4 with improved rate performance and cyclic stability for supercapacitors. Chem Res Chinese Universities 36:68–73. https://doi.org/10.1007/s40242-019-0040-3
Zhao J, Wang J, Bi R et al (2020) General synthesis of multiple-cores@ multiple-shells hollow composites and their application to lithium–ion batteries. Angew Chem Int Ed 60:25719–25722. https://doi.org/10.1007/s40242-019-0040-3
Zhao J, Yang M, Yang N et al (2020) Hollow micro-/nanostructure reviving lithium-sulfur batteries. Chem Res Chinese Universities 36:313–319. https://doi.org/10.1007/s40242-020-0115-2
Wang J, Wan J, Yang N et al (2020) Hollow multishell structures exercise temporal-spatial ordering and dynamic smart behaviour. Nat Rev Chem 4:159–168. https://doi.org/10.1038/s41570-020-0161-8
Wei Y, Wan J, Yang N et al (2020) Efficient sequential harvesting of solar light by heterogeneous hollow shells with hierarchical pores. Natl Sci Rev 7:1638–1646. https://doi.org/10.1093/nsr/nwaa059
Mao D, Wan J, Wang J et al (2018) Sequential templating approach: a groundbreaking strategy to create hollow multishelled structures. Adv Mater 31:1802874. https://doi.org/10.1002/adma.201802874
Holzwarth U, Gibson N (2011) The Scherrer equation versus the’Debye-Scherrer equation’. Nat Nanotechnol 6:534–534. https://doi.org/10.1038/nnano.2011.145
ElBellihi AA, Bayoumy WA, Masoud EM (2012) Preparation, characterizations and conductivity of composite polymer electrolytes based on PEO-LiClO4 and Nano ZnO filler. B Korean Chem Soc 33:2949–2954. https://doi.org/10.5012/bkcs.2012.33.9.2949
Paranjape N, Mandadapu PC, Wu G et al (2017) Highly-branched cross-linked poly (ethylene oxide) with enhanced ionic conductivity. Polymer 111:1–8. https://doi.org/10.1016/j.polymer.2017.01.014
Appetecchi GB, Henderson W, Villano P et al (2001) PEO-LiN(SO2CF2CF3)2 Polymer electrolytes: I. XRD, DSC, and ionic conductivity characterization. J Electrochem Soc 148:A1171–A1178. https://doi.org/10.1149/1.1403728
Li Y, Yu YC, Han CY et al (2020) Sustainable blends of poly (propylene carbonate) and stereocomplex polylactide with enhanced rheological properties and heat resistance. Chinese J Polym Sci 38:1267–1275. https://doi.org/10.1007/s10118-020-2408-8
Stolwijk NA, Heddier C, Reschke M et al (2013) Salt-concentration dependence of the glass transition temperature in PEO-NaI and PEO-LiTFSI polymer electrolytes. Macromolecules 46:8580–8588. https://doi.org/10.1021/ma401686r
Homann G, Stolz L, Nair J et al (2020) Poly (ethylene oxide)-based electrolyte for solid-state-lithium-batteries with high voltage positive electrodes: evaluating the role of electrolyte oxidation in rapid cell failure. Sci Rep 10:1–9. https://doi.org/10.1038/s41598-020-61373-9
Ma Y, Wan J, Yang Y et al (2022) Scalable, ultrathin, and high-temperature-resistant solid polymer electrolytes for energy-dense lithium metal batteries. Adv Energy Mater 12:2103720. https://doi.org/10.1002/aenm.202103720
Qiu J, Liu X, Chen R et al (2020) Enabling stable cycling of 4.2 V high-voltage all-solid-state batteries with PEO-based solid electrolyte. Adv Funct Mater 30:1909392. https://doi.org/10.1002/adfm.201909392
Jiao Y, Li F, Jin X et al (2021) Engineering polymer glue towards 90% zinc utilization for 1000 hours to make high-performance Zn-Ion batteries. Adv Funct Mater 31:2107652. https://doi.org/10.1002/adfm.202107652
Liu Y, Zhao Y, Lu W et al (2021) PEO based polymer in plastic crystal electrolytes for room temperature high-voltage lithium metal batteries. Nano Energy 88:106205. https://doi.org/10.1016/j.nanoen.2021.106205
Acknowledgements
We are grateful to Professor Kai Liu’s laboratory of Tsinghua University for the technical guidance of sample preparation provided in this study.
Funding
This work was financially supported by the Natural Science Foundation of China (Grant No.: 51932001, 51872024, 21820102002, 21931012, 22111530178, 51972305, 51972306), the National Key R&D Program (Grant No.: 2018YFA0703503, 2021YFC2902500, 2022YFA1504100), the Cooperation Fund of the Institute of Clean Energy Innovation, Chinese Academy of Sciences (Grant No.: DNL202020), the Zhongke-Yuneng Joint R&D Center Program (No.: ZKYN2022008).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. D.W., R.Y., and J.W. conceived the idea and supervised the research. Y.M. performed experiments and basic characterizations. D.W., R.Y., J.W., Y.M., R.B., M.Y., J.Q., and P.W. analyzed and discussed the experimental data. Y.M. and J.W. drafted the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the topical collection: Self-assembled Functional Nanomaterials and Devices in Asia
Guest Editosr: Zhixiang Wei and Yong Yan
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, Y., Bi, R., Yang, M. et al. Hollow multishelled structural ZnO fillers enhance the ionic conductivity of polymer electrolyte for lithium batteries. J Nanopart Res 25, 14 (2023). https://doi.org/10.1007/s11051-022-05661-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-022-05661-7