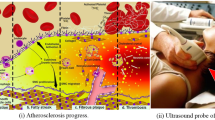
Abstract
Atherosclerosis, caused by a variety of extrinsic risk factors, is the major cause of the cardiovascular and cerebrovascular diseases that bring high mortality and morbidity globally, with substantial socio-economic impact. The development and progression of atherosclerosis is insidious, and early detection and intervention is vital to prevent debilitating cardiovascular events like myocardial infarction and stroke. Imaging biomarkers like carotid intima-media thickness (cIMT) and plaque area/burden that can identify subclinical disease and stratify risk are therefore crucial. This comprehensive review focuses on these biomarkers, their imaging using ultrasound, assessment using machine learning and deep learning techniques, and their association with cardiovascular risk factors. Measurement of cIMT and plaque area/burden in the common carotid artery (CCA) using deep learning models built on convolutional neural networks like UNet have shown good accuracy and reliability compared to manual methods. Expanding the scope to include the internal carotid artery (ICA) and carotid bulb presents greater technical challenges due to image acquisition difficulties, but deep learning methods utilizing architectures like attention-UNet show promise. However, several biases exist in current deep learning systems stemming from limited multi-center datasets, as models trained on specific patient cohorts underperform when assessed on diverse unseen test data. Eliminating these biases through techniques like transfer learning, aggregating multi-ethnic data, comparing multiple models, and combining deep learning with optimization algorithms can make these AI systems more generalizable and robust. Moving forward, automated measurement of carotid ultrasound imaging biomarkers of subclinical atherosclerosis using bias-free deep learning approaches can enable large-scale screening to identify individuals at risk of cardiovascular events, allowing early intervention to modify risk and prevent disease progression.










Similar content being viewed by others
Data availability
There is no database associated with this study.
References
Virani SS et al (2021) Heart disease and stroke statistics-2021 update: a report from the american heart association (in eng). Circulation 143(8):e254–e743. https://doi.org/10.1161/cir.0000000000000950
Cardiovascular diseases (CVDs) https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). Accessed 20 Jul 2022
Dunbar SB et al (2018) Projected costs of informal caregiving for cardiovascular disease: 2015 to 2035: a policy statement from the American Heart Association. Circulation 137(19):e558–e577. https://doi.org/10.1161/CIR.0000000000000570
Kamalakannan S, Gudlavalleti AS, Gudlavalleti VSM, Goenka S, Kuper H (2017) Incidence & prevalence of stroke in India: a systematic review. Indian J Med Res 146(2):175. https://doi.org/10.4103/ijmr.IJMR_516_15
Chauhan S, Aeri BT (2015) The rising incidence of cardiovascular diseases in India: assessing its economic impact. J Prev Cardiol 4(4):735–40, https://api.semanticscholar.org/CorpusID:34741854.
Prabhakaran D, Jeemon P, Roy A (2016) Cardiovascular diseases in India: current epidemiology and future directions. Circulation 133(16):1605–1620. https://doi.org/10.1161/circulationaha.114.008729
Suri JS, Kathuria C, Molinari F (2010) Atherosclerosis disease management. Springer-Verlag New York Inc, p 944–1250. https://doi.org/10.1007/978-1-4419-7222-4, 2011
Park TH (2016) Evaluation of carotid plaque using ultrasound imaging. J Cardiovasc Ultrasound 24(2):91–95. https://doi.org/10.4250/jcu.2016.24.2.91
Patel AK et al (2016) A review on atherosclerotic biology, wall stiffness, physics of elasticity, and its ultrasound-based measurement. Curr Atheroscler Rep 18(12):1–12. https://doi.org/10.1007/s11883-016-0635-9
Barnett HJM et al. (1998) Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. N Engl J Med 339(20):1415–1425. https://cir.nii.ac.jp/crid/1573105975361337216. Accessed 02 Apr 2023
Naim C et al (2014) Vulnerable atherosclerotic carotid plaque evaluation by ultrasound, computed tomography angiography, and magnetic resonance imaging: an overview. Can Assoc Radiol J 65(3):275–286. https://doi.org/10.1016/j.carj.2013.05.003
Roy S, Chatterjee K, Bandyopadhyay SK (2014) Segmentation of acute brain stroke from MRI of brain image using power law transformation with accuracy estimation. In: Kumar Kundu M, Mohapatra D, Konar A, Chakraborty A (eds) Advanced computing, networking and informatics, vol 1. Smart innovation, systems and technologies, vol 27. Springer, Cham. https://doi.org/10.1007/978-3-319-07353-8_53
Libby P (2006) Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr 83(2):456S-460S. https://doi.org/10.1093/ajcn/83.2.456S
Naghavi M et al (2003) From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation 108(14):1664–1672. https://doi.org/10.1161/01.CIR.0000087480.94275.97
Hopkins PN (2013) Molecular biology of atherosclerosis. Physiol Rev https://doi.org/10.1152/physrev.00004.2012
Libby P (2006) Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr 83(2):456S-460S. https://doi.org/10.1093/ajcn/83.2.456s
Togay-Isikay C et al (2005) Carotid artery tortuosity, kinking, coiling: stroke risk factor, marker, or curiosity? Acta Neurol Belg 105(2):68. https://pubmed.ncbi.nlm.nih.gov/16076059. Accessed 02 Apr 2023
Mannarino E, Pirro M (2008) Molecular biology of atherosclerosis. Clin Cases Miner Bone Metab 5(1):57. https://pubmed.ncbi.nlm.nih.gov/22460847. Accessed 02 Apr 2023
Roy S, Meena T, Lim S-J (2022) Demystifying supervised learning in healthcare 4.0: A new reality of transforming diagnostic medicine. Diagnostics 12(10):2549. https://doi.org/10.3390/diagnostics12102549
Ho SSY (2016) Current status of carotid ultrasound in atherosclerosis. Quant Imaging Med Surg 6(3):285
Libby P, Ridker PM, Hansson GK (2009) Inflammation in Atherosclerosis: From Pathophysiology to Practice. J Am Coll Cardiol 54(23):2129–2138. https://doi.org/10.1016/j.jacc.2009.09.009
Picano E, Paterni M (2015) Ultrasound tissue characterization of vulnerable atherosclerotic plaque. Int J Mol Sci 16(5):10121–10133. https://doi.org/10.3390/ijms160510121
Roy S, Bhattacharyya D, Bandyopadhyay SK, Kim T-H (2017) An effective method for computerized prediction and segmentation of multiple sclerosis lesions in brain MRI. Comput Methods Programs Biomed 140:307–320. https://doi.org/10.1016/j.cmpb.2017.01.003
Rothwell PM, Warlow CP (2000) Low risk of ischemic stroke in patients with reduced internal carotid artery lumen diameter distal to severe symptomatic carotid stenosis: cerebral protection due to low poststenotic flow? Stroke 31(3):622–630. https://doi.org/10.1161/01.str.31.3.622
Mathiesen EB, Johnsen SH, Wilsgaard T, Bønaa KH, Løchen M-L, Njølstad I (2011) Carotid plaque area and intima-media thickness in prediction of first-ever ischemic stroke: a 10-year follow-up of 6584 men and women: the Tromsø study. Stroke 42(4):972–978. https://doi.org/10.1161/strokeaha.110.589754
Liu Y et al (2019) Size of carotid artery intraplaque hemorrhage and acute ischemic stroke: a cardiovascular magnetic resonance chinese atherosclerosis risk evaluation study. J Cardiovasc Magn Reson 21(1):1–9. https://doi.org/10.1186/s12968-019-0548-1
Acharya UR et al (2012) Plaque tissue characterization and classification in ultrasound carotid scans: a paradigm for vascular feature amalgamation. IEEE Trans Instrum Meas 62(2):392–400. https://doi.org/10.1109/TIM.2012.2217651
Dong Y, Pan Y, Zhao X, Li R, Yuan C, Xu W (2017) Identifying carotid plaque composition in MRI with convolutional neural networks. In: IEEE International Conference on Smart Computing (SMARTCOMP). IEEE, pp 1–8. https://doi.org/10.1109/SMARTCOMP.2017.7947015
Gupta A et al (2015) Plaque echolucency and stroke risk in asymptomatic carotid stenosis: a systematic review and meta-analysis. Stroke 46(1):91–97. https://doi.org/10.1161/strokeaha.114.006091
Mohebali J et al (2015) Acoustic shadowing impairs accurate characterization of stenosis in carotid ultrasound examinations. J Vasc Surg 62(5):1236–1244. https://doi.org/10.1016/j.jvs.2015.06.137
Hunt KJ et al (2001) Acoustic shadowing on B-mode ultrasound of the carotid artery predicts ischemic stroke: the atherosclerosis risk in communities (ARIC) study. Stroke 32(5):1120–1126. https://doi.org/10.1161/01.str.32.5.1120
Saba L et al (2018) Intra-and inter-operator reproducibility analysis of automated cloud-based carotid intima media thickness ultrasound measurement. J Clin Diagn Res: JCDR 12(2). https://doi.org/10.1016/j.ihj.2018.01.024
Saba L et al (2018) Intra-and inter-operator reproducibility of automated cloud-based carotid lumen diameter ultrasound measurement. Indian Heart J 70(5):649–664. https://doi.org/10.1016/j.ihj.2018.01.024
Kim G-H, Youn H-J (2017) Is carotid artery ultrasound still useful method for evaluation of atherosclerosis? Korean Circ J 47(1):1–8. https://doi.org/10.4070/kcj.2016.0232
Chiu B, Shamdasani V, Entrekin R, Yuan C, Kerwin WS (2012) Characterization of carotid plaques on 3-dimensional ultrasound imaging by registration with multicontrast magnetic resonance imaging. J Ultrasound Med 31(10):1567–1580. https://doi.org/10.7863/jum.2012.31.10.1567
de Korte CL, Fekkes S, Nederveen AJ, Manniesing R, Hansen HRHG (2016) Mechanical characterization of carotid arteries and atherosclerotic plaques. IEEE Trans Ultrason Ferroelectr Freq Control 63(10):1613–1623. https://doi.org/10.1109/tuffc.2016.2572260
Chu B et al (2004) Hemorrhage in the atherosclerotic carotid plaque: a high-resolution MRI study. Stroke 35(5):1079–1084. https://doi.org/10.1161/01.str.0000125856.25309.86
Kamenskiy AV, Pipinos II, Carson JS, MacTaggart JN, Baxter BT (2015) Age and disease-related geometric and structural remodeling of the carotid artery. J Vasc Surg 62(6):1521–1528. https://doi.org/10.1016/j.jvs.2014.10.041
Saba L et al (2021) Review of imaging biomarkers for the vulnerable carotid plaque. JVS-Vasc Sci 2:149–158. https://doi.org/10.1016/j.jvssci.2021.03.001
Benjamin EJ et al (2019) Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation 139(10):e56–e528. https://doi.org/10.1161/cir.0000000000000659
Tracqui P, Broisat A, Toczek J, Mesnier N, Ohayon J, Riou L (2011) Mapping elasticity moduli of atherosclerotic plaque in situ via atomic force microscopy. J Struct Biol 174(1):115–123. https://doi.org/10.1016/j.jsb.2011.01.010
Teng Z et al (2014) Material properties of components in human carotid atherosclerotic plaques: a uniaxial extension study. Acta Biomater 10(12):5055–5063. https://doi.org/10.1016/j.actbio.2014.09.001
Mirek AM, Wolińska-Welcz A (2013) Is the lumen diameter of peripheral arteries a good marker of the extent of coronary atherosclerosis? Kardiol Polska (Pol Heart J) 71(8):810–817. https://doi.org/10.5603/kp.2013.0192
Nambi V, Brunner G, Ballantyne CM (2013) Ultrasound in cardiovascular risk prediction: don’t forget the plaque! Am Heart Assoc 2:e000180. https://doi.org/10.1161/jaha.113.000180
Remington LA, Goodwin D (2021) Clinical anatomy and physiology of the visual system E-Book. Elsevier Health Sciences, p 350
Londhe ND, Suri JS (2016) Superharmonic imaging for medical ultrasound: a review. Trans Process Syst 40(12):1–16. https://doi.org/10.1007/s10916-016-0635-x
Jashari F, Ibrahimi P, Bajraktari G, Grönlund C, Wester P, Henein MY (2016) Carotid plaque echogenicity predicts cerebrovascular symptoms: a systematic review and meta-analysis (in eng). Eur J Neurol 23(7):1241–1247. https://doi.org/10.1111/ene.13017
Özdemir H, Artas H, Serhatlioglu S, Ogur E (2006) Effects of overweight on luminal diameter, flow velocity and intima-media thickness of carotid arteries. Diagn Interv Radiol 12(3):142. https://pubmed.ncbi.nlm.nih.gov/16972220/. Accessed 15 Apr 2023
Lloyd KD, Barinas-Mitchell E, Kuller LH, Mackey RH, Wong EA, Sutton-Tyrrell K (2012) Common carotid artery diameter and cardiovascular risk factors in overweight or obese postmenopausal women. Int J Vasc Med 2012, https://doi.org/10.1155/2012/169323
Krejza J et al (2006) Carotid artery diameter in men and women and the relation to body and neck size. Stroke 37(4):1103–1105. https://doi.org/10.1161/01.str.0000206440.48756.f7
Polak JF, O’Leary DH (2016) Carotid intima-media thickness as surrogate for and predictor of CVD. Global Heart 11(3):295-312.e3. https://doi.org/10.1016/j.gheart.2016.08.006
Ruan L et al (2009) Correlates of common carotid artery lumen diameter in black and white younger adults: the Bogalusa heart study. Stroke 40(3):702–707. https://doi.org/10.1161/strokeaha.108.531608
Mancini GBJ, Dahlöf BR, Diez J (2004) Surrogate markers for cardiovascular disease: structural markers. Circulation 109(25_suppl_1):IV–22. https://doi.org/10.1161/01.cir.0000133443.77237.2f
Cohn JN, Quyyumi AA, Hollenberg NK, Jamerson KA (2004) Surrogate markers for cardiovascular disease: functional markers. Circulation 109(25_suppl_1):IV–31. https://doi.org/10.1161/01.cir.0000133442.99186.39
Amato M et al (2007) Carotid intima-media thickness by B-mode ultrasound as surrogate of coronary atherosclerosis: correlation with quantitative coronary angiography and coronary intravascular ultrasound findings. Eur Heart J 28(17):2094–2101. https://doi.org/10.1093/eurheartj/ehm244
Hong JT et al (2010) The effect of patient age on the internal carotid artery location around the atlas. J Neurosurg Spine 12(6):613–618. https://doi.org/10.3171/2010.1.spine09409
Bartlett ES, Symons SP, Fox AJ (2006) Correlation of carotid stenosis diameter and cross-sectional areas with CT angiography. Am J Neuroradiol 27(3):638–642. https://pubmed.ncbi.nlm.nih.gov/16552008. Accessed 17 Mar 2023
Hyde DE et al (2004) Internal carotid artery stenosis measurement: comparison of 3D computed rotational angiography and conventional digital subtraction angiography. Stroke 35(12):2776–2781. https://doi.org/10.1161/01.str.0000147037.12223.d5
Pleouras DS et al (2022) Prediction of the atherosclerotic plaque development in carotid arteries; the effect of T-cells. In: 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC). IEEE, pp 1590–1593. https://doi.org/10.1109/embc48229.2022.9871632
Saba L et al (2019) Global perspective on carotid intima-media thickness and plaque: should the current measurement guidelines be revisited? Int Angiol. https://doi.org/10.23736/s0392-9590.19.04267-6
Saba L et al (2017) Plaque tissue morphology-based stroke risk stratification using carotid ultrasound: a polling-based PCA learning paradigm. J Med Syst 41(6):98. https://doi.org/10.1007/s10916-017-0745-0
Araki T et al (2017) Stroke risk stratification and its validation using ultrasonic echolucent carotid wall plaque morphology: a machine learning paradigm. Comput Biol Med 80:77–96. https://doi.org/10.1016/j.compbiomed.2016.11.011
Saba L et al (2016) Carotid inter-adventitial diameter is more strongly related to plaque score than lumen diameter: an automated tool for stroke analysis. J Clin Ultrasound 44(4):210–220. https://doi.org/10.1002/jcu.22334
Krishna Kumar P et al (2017) Accurate lumen diameter measurement in curved vessels in carotid ultrasound: an iterative scale-space and spatial transformation approach (in eng). Med Biol Eng Comput 55(8):1415–1434. https://doi.org/10.1007/s11517-016-1601-y
Bots ML et al (2007) Carotid intima-media thickness and coronary atherosclerosis: weak or strong relations? Eur Heart J 28(4):398–406. https://doi.org/10.1093/eurheartj/ehl482
Molinari F, Zeng G, Suri JS (2010) Intima-media thickness: setting a standard for a completely automated method of ultrasound measurement. IEEE Trans Ultrason Ferroelectr Freq Control 57(5):1112–1124. https://doi.org/10.1109/tuffc.2010.1522
Molinari F et al (2012) Completely automated multiresolution edge snapper–a new technique for an accurate carotid ultrasound IMT measurement: clinical validation and benchmarking on a multi-institutional database. IEEE Trans Image Process 21(3):1211–1222. https://doi.org/10.1109/tip.2011.2169270
Cuadrado-Godia E et al (2018) Geometric total plaque area is an equally powerful phenotype compared with carotid intima-media thickness for stroke risk assessment: a deep learning approach. J Vasc Ultrasound 42(4):162–188. https://doi.org/10.1177/1544316718806421
Cuadrado-Godia E et al (2018) Morphologic TPA (mTPA) and composite risk score for moderate carotid atherosclerotic plaque is strongly associated with HbA1c in diabetes cohort. Comput Biol Med 101:128–145. https://doi.org/10.1016/j.compbiomed.2018.08.008
Herder M, Johnsen SH, Arntzen KA, Mathiesen EB (2012) Risk factors for progression of carotid intima-media thickness and total plaque area: a 13-year follow-up study: the Tromsø study. Stroke 43(7):1818–1823. https://doi.org/10.1161/strokeaha.111.646596
Kamycheva E, Johnsen SH, Wilsgaard T, Jorde R, Mathiesen EB (2013) Evaluation of serum 25-hydroxyvitamin D as a predictor of carotid intima-media thickness and carotid total plaque area in nonsmokers: the Tromsø Study. Int J Endocrinol 2013. https://doi.org/10.1155/2013/305141
Spence JD, Hegele RA (2004) Noninvasive phenotypes of atherosclerosis. Arterioscler Thromb Vasc Biol 24(11):e188–e189. https://doi.org/10.1161/01.atv.0000146160.22637.33
Cau R et al (2021) Artificial intelligence in computed tomography plaque characterization: A review. Eur J Radiol 109767. https://doi.org/10.1016/j.ejrad.2021.109767
Lee W (2014) General principles of carotid Doppler ultrasonography. Ultrasonography 33(1):11. https://doi.org/10.14366/usg.13018
Molinari F, Meiburger KM, Zeng G, Nicolaides A, Suri JS (2012) CAUDLES-EF: carotid automated ultrasound double line extraction system using edge flow. In: Ultrasound Imaging. Springer, pp 129–162. https://doi.org/10.1007/s10278-011-9375-0
Saba L et al (2016) Accurate cloud-based smart IMT measurement, its validation and stroke risk stratification in carotid ultrasound: a web-based point-of-care tool for multicenter clinical trial. Comput Biol Med 75:217–234. https://doi.org/10.1016/j.compbiomed.2016.06.010
Molinari F et al (2011) CARES 3.0: a two stage system combining feature-based recognition and edge-based segmentation for CIMT measurement on a multi-institutional ultrasound database of 300 images. In: 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE, pp 5149–5152. https://doi.org/10.1109/iembs.2011.6091275
Biswas M et al (2019) Deep learning fully convolution network for lumen characterization in diabetic patients using carotid ultrasound: a tool for stroke risk. Med Biol Eng Comput 57(2):543–564. https://doi.org/10.1007/s11517-018-1897-x
Cootes TF, Taylor CJ, Cooper DH, Graham J (1995) Active shape models-their training and application. Comput Vis Image Underst 61(1):38–59. https://doi.org/10.1006/cviu.1995.1004
Balakrishnan S, Joseph PK (2022) Stratification of risk of atherosclerotic plaque using Hu’s moment invariants of segmented ultrasonic images. Biomed Eng/Biomed Tech 67(5):391–402. https://doi.org/10.1515/bmt-2021-0044
Araki T et al (2016) PCA-based polling strategy in machine learning framework for coronary artery disease risk assessment in intravascular ultrasound: a link between carotid and coronary grayscale plaque morphology. Comput Methods Prog Biomed 128:137–158. https://doi.org/10.1016/j.cmpb.2016.02.004
Araki T et al (2016) A new method for IVUS-based coronary artery disease risk stratification: a link between coronary & carotid ultrasound plaque burdens. Comput Methods Prog Biomed 124:161–179. https://doi.org/10.1016/j.cmpb.2015.10.022
Roy S, Shoghi KI (2019) Computer-aided tumor segmentation from T2-weighted MR images of patient-derived tumor xenografts. In: Image analysis and recognition: 16th International conference, ICIAR 2019, Waterloo, ON, Canada, August 27–29, 2019, proceedings, part II 16. Springer International Publishing, pp 159–171. https://doi.org/10.1007/978-3-030-27272-2_14
Gibson E et al (2018) NiftyNet: a deep-learning platform for medical imaging. Comput Methods Programs Biomed 158:113–122. https://doi.org/10.1016/j.cmpb.2018.01.025
Zreik M, Van Hamersvelt RW, Wolterink JM, Leiner T, Viergever MA, Išgum I (2018) A recurrent CNN for automatic detection and classification of coronary artery plaque and stenosis in coronary CT angiography. IEEE Trans Med Imaging 38(7):1588–1598. https://doi.org/10.1109/tmi.2018.2883807
Biswas M et al (2018) Deep learning strategy for accurate carotid intima-media thickness measurement: an ultrasound study on Japanese diabetic cohort. Comput Biol Med 98:100–117. https://doi.org/10.1016/j.compbiomed.2018.05.014
Kb J, Madian N (2018) Convolutional neural network for segmentation and measurement of intima media thickness. J Med Syst 42(8):1–8. https://doi.org/10.1007/s10916-018-1001-y
Menchón-Lara R-M, Sancho-Gómez J-L, Bueno-Crespo A (2016) Early-stage atherosclerosis detection using deep learning over carotid ultrasound images. Appl Soft Comput 49:616–628. https://doi.org/10.1016/j.asoc.2016.08.055
Gangopadhyay T, Halder S, Dasgupta P, Chatterjee K, Ganguly D, Sarkar S, Roy S (2022) MTSE U-Net: an architecture for segmentation, and prediction of fetal brain and gestational age from MRI of brain. Netw Model Anal Health Inf Bioinform 11(1):50. https://doi.org/10.1007/s13721-022-00394-y
Liu S et al (2019) Deep learning in medical ultrasound analysis: a review. Engineering 5(2):261–275. https://doi.org/10.1016/j.eng.2018.11.020
Halder S et al (2023) Fetal brain component segmentation using 2-way ensemble U-Net. In: Sharma N, Goje A, Chakrabarti A, Bruckstein AM (eds) Data management, analytics and innovation. ICDMAI 2023. Lecture notes in networks and systems, vol 662. Springer, Singapore. https://doi.org/10.1007/978-981-99-1414-2_28
Kabiraj A, Meena T, Reddy PB, Roy S (2022) Detection and classification of lung disease using deep learning architecture from x-ray images. In: International symposium on visual computing. Springer International Publishing, Cham, pp 444–455. https://doi.org/10.1007/978-3-031-20713-6_34
Meyer P, Noblet V, Mazzara C, Lallement A (2018) Survey on deep learning for radiotherapy. Comput Biol Med 98:126–146. https://doi.org/10.1016/j.compbiomed.2018.05.018
Sharma N, Kalra M, Viskovic K, Saba L, Suri J (2022) Four types of multiclass frameworks for pneumonia classification and its validation in x-ray scans using seven types of deep learning artificial intelligence models. Diagnostics 12(652). https://doi.org/10.3390/diagnostics12030652
Wei J et al (2021) Genetic U-Net: Automatically designed deep networks for retinal vessel segmentation using a genetic algorithm. IEEE Trans Med Imaging. https://doi.org/10.1109/TMI.2021.3111679
del Mar Vila M et al (2020) Semantic segmentation with DenseNets for carotid artery ultrasound plaque segmentation and CIMT estimation. Artif Intell Med 103:101784. https://doi.org/10.1016/j.artmed.2019.101784
Zhang Z, Wu C, Coleman S, Kerr D (2020) DENSE-INception U-net for medical image segmentation. Comput Methods Programs Biomed 192:105395. https://doi.org/10.1016/j.cmpb.2020.105395
Punn NS, Agarwal S (2020) Inception u-net architecture for semantic segmentation to identify nuclei in microscopy cell images. ACM Trans Multimed Comput Commun Appl 16(1):1–15. https://doi.org/10.1145/3376922
Park JH, Seo E, Choi W, Lee SJ (2022) Ultrasound deep learning for monitoring of flow–vessel dynamics in murine carotid artery. Ultrasonics 120:106636. https://doi.org/10.1016/j.ultras.2021.106636
Balaha HM, Balaha MH, Ali HA (2021) Hybrid COVID-19 segmentation and recognition framework (HMB-HCF) using deep learning and genetic algorithms. Artif Intell Med 119:102156. https://doi.org/10.1016/j.artmed.2021.102156
Zhou R et al (2021) Deep learning-based carotid plaque segmentation from B-mode ultrasound images. Ultrasound Med Biol 47(9):2723–2733. https://doi.org/10.1016/j.ultrasmedbio.2021.05.023
Jain PK et al (2021) Automated deep learning-based paradigm for high-risk plaque detection in B-mode common carotid ultrasound scans: an asymptomatic Japanese cohort study. Int Angiol https://doi.org/10.23736/s0392-9590.21.04771-4
Liapi GD, Kyriacou E, Loizou CP, Panayides AS, Pattichis CS, Nicolaides AN (2022) Deep learning-based segmentation of the atherosclerotic carotid plaque in ultrasonic images. Springer, pp 187–198. https://doi.org/10.1007/978-3-031-08341-9_16
Li Y et al (2022) FRDD-Net: Automated carotid plaque ultrasound images segmentation using feature remapping and dense decoding. Sensors 22(3):887. [Online]. Available: https://www.mdpi.com/1424-8220/22/3/887. Accessed 12 Apr 2023
Jain PK, Sharma N, Giannopoulos AA, Saba L, Nicolaides A, Suri JS (2021) Hybrid deep learning segmentation models for atherosclerotic plaque in internal carotid artery B-mode ultrasound. Comput Biol Med 136:104721. https://doi.org/10.1016/j.compbiomed.2021.104721
Zhou R et al (2021) Deep learning-based measurement of total plaque area in B-mode ultrasound images. IEEE J Biomed Health Inform. https://doi.org/10.1109/JBHI.2021.3060163
Jain PK, Sharma N, Kalra MK, Johri A, Saba L, Suri JS (2022) Far wall plaque segmentation and area measurement in common and internal carotid artery ultrasound using U-series architectures: An unseen Artificial Intelligence paradigm for stroke risk assessment. Comput Biol Med 106017. https://doi.org/10.1016/j.compbiomed.2022.106017
Meshram NH, Mitchell CC, Wilbrand S, Dempsey RJ, Varghese T (2020) Deep learning for carotid plaque segmentation using a dilated U-net architecture. Ultrason Imaging 42(4–5):221–230. https://doi.org/10.1177/0161734620951216
Yang J, Faraji M, Basu A (2019) Robust segmentation of arterial walls in intravascular ultrasound images using dual path U-Net. Ultrasonics 96:24–33. https://doi.org/10.1016/j.ultras.2019.03.014
Biswas M et al (2020) Two-stage artificial intelligence model for jointly measurement of atherosclerotic wall thickness and plaque burden in carotid ultrasound: a screening tool for cardiovascular/stroke risk assessment. Comput Biol Med 123:103847. https://doi.org/10.1016/j.compbiomed.2020.103847
Zhou T, Tan T, Pan X, Tang H, Li J (2021) Fully automatic deep learning trained on limited data for carotid artery segmentation from large image volumes. Quant Imaging Med Surg 11(1):67. https://doi.org/10.21037/qims-20-286
Ma W et al (2022) Object-specific four-path network for stroke risk stratification of carotid arteries in ultrasound images. Comput Math Methods Med 2022:2014349. https://doi.org/10.1155/2022/2014349
Jain PK et al (2021) Unseen artificial intelligence—Deep learning paradigm for segmentation of low atherosclerotic plaque in carotid ultrasound: a multicenter cardiovascular study. Diagnostics 11(12):2257. https://doi.org/10.3390/diagnostics11122257
Zhou Z, Siddiquee MMR, Tajbakhsh N, Liang J (2019) Unet++: redesigning skip connections to exploit multiscale features in image segmentation. IEEE Trans Med Imaging 39(6):1856–1867. https://doi.org/10.1109/tmi.2019.2959609
Mohamed AAA, Hançerlioğullari A, Rahebi J, Ray MK, Roy S (2023) Colon disease diagnosis with convolutional neural network and grasshopper optimization algorithm. Diagnostics 13(10):1728
Zhou R, Fenster A, Xia Y, Spence JD, Ding M (2019) Deep learning-based carotid media-adventitia and lumen-intima boundary segmentation from three-dimensional ultrasound images. Med Phys 46(7):3180–3193. https://doi.org/10.1002/mp.13581
Lakshmi Prabha P, Jayanthy A, Prem Kumar C, Ramraj B (2021) Prediction of cardiovascular risk by measuring carotid intima media thickness from an ultrasound image for type II diabetic mellitus subjects using machine learning and transfer learning techniques. J Supercomput 77(9):10289–10306. https://doi.org/10.1007/s11227-021-03676-w
Meiburger KM et al (2022) Carotid ultrasound boundary study (CUBS): technical considerations on an open multi-center analysis of computerized measurement systems for intima-media thickness measurement on common carotid artery longitudinal B-mode ultrasound scans. Computers in Biology Medicine 144:105333. https://doi.org/10.1016/j.compbiomed.2022.105333
Tajbakhsh N, Shin JY, Hurst RT, Kendall CB, Liang J (2017) Automatic interpretation of carotid intima–media thickness videos using convolutional neural networks. In: Deep learning for medical image analysis. Elsevier, pp 105–131. https://doi.org/10.1016/B978-0-12-810408-8.00007-9
Zhou Z, Shin J, Feng R, Hurst RT, Kendall CB, Liang J (2019) Integrating active learning and transfer learning for carotid intima-media thickness video interpretation. J Digit Imaging 32(2):290–299. https://doi.org/10.1007/s10278-018-0143-2
Lin Y, Huang J, Chen Y, Chen Q, Li Z, Cao Q (2022) Intelligent segmentation of intima–media and plaque recognition in carotid artery ultrasound images. Ultrasound Med Biol 48(3):469–479. https://doi.org/10.1016/j.ultrasmedbio.2021.11.001
Gago L, del Mar Vila M, Grau M, Remeseiro B, Igual L (2022) An end-to-end framework for intima media measurement and atherosclerotic plaque detection in the carotid artery. Comput Methods Programs Biomed 223:106954. https://doi.org/10.1016/j.cmpb.2022.106954
Saba L et al (2021) A multicenter study on carotid ultrasound plaque tissue characterization and classification using six deep artificial intelligence models: a stroke application. IEEE Trans Instrum Meas 70:1–12. https://doi.org/10.1109/TIM.2021.3052577
Skandha SS et al (2022) A hybrid deep learning paradigm for carotid plaque tissue characterization and its validation in multicenter cohorts using a supercomputer framework. Computers in Biology Medicine 141:105131. https://doi.org/10.1016/j.compbiomed.2021.105131
Lekadir K et al (2017) "A convolutional neural network for automatic characterization of plaque composition in carotid ultrasound (in eng). IEEE J Biomed Health Inform 21(1):48–55. https://doi.org/10.1109/jbhi.2016.2631401
Khanna NN et al (2019) Rheumatoid arthritis: atherosclerosis imaging and cardiovascular risk assessment using machine and deep learning–based tissue characterization. Curr Atheroscler Rep 21(2):1–14. https://doi.org/10.1007/s11883-019-0766-x
Boi A et al (2018) A survey on coronary atherosclerotic plaque tissue characterization in intravascular optical coherence tomography. Curr Atheroscler Rep 20(7):1–17. https://doi.org/10.1007/s11883-018-0736-8
Li R, Zheng J, Zayed MA, Saffitz JE, Woodard PK, Jha AK (2023) Carotid atherosclerotic plaque segmentation in multi-weighted MRI using a two-stage neural network: advantages of training with high-resolution imaging and histology. Front Cardiovasc Med 10:1127653. https://doi.org/10.3389/fcvm.2023.1127653
Zhu Y, Chen L, Lu W, Gong Y, Wang X (2022) The application of the nnU-net-based automatic segmentation model in assisting carotid artery stenosis and carotid atherosclerotic plaque evaluation. Front Physiol 13:2484. https://doi.org/10.3389/fphys.2022.1057800
Singh S, Jain PK, Sharma N, Pohit M, Roy S (2023) Atherosclerotic plaque classification in carotid ultrasound images using machine learning and explainable deep learning,". Intell Med. https://doi.org/10.1016/j.imed.2023.05.003
Pal D, Meena T, Roy S (2023) A fully connected reproducible SE-UResNet for multiorgan chest radiographs segmentation. In: 2023 IEEE 24th international conference on information reuse and integration for data science (IRI), Bellevue, WA, USA, pp 261–266. https://doi.org/10.1109/IRI58017.2023.00052
Meena T, Kabiraj A, Reddy PB, Roy S (2023) Weakly supervised confidence aware probabilistic CAM multi-thorax anomaly localization network. In: 2023 IEEE 24th international conference on information reuse and integration for data science (IRI), Bellevue, WA, USA, pp 309–314. https://doi.org/10.1109/IRI58017.2023.00061
Pal D, Reddy PB, Roy S (2022) Attention UW-net: a fully connected model for automatic segmentation and annotation of chest X-ray. Comput Biol Med 150:106083. https://doi.org/10.1016/j.compbiomed.2022.106083
Jain PK et al (2022) Attention-based UNet deep learning model for plaque segmentation in carotid ultrasound for stroke risk stratification: an artificial intelligence paradigm. J Cardiovasc Dev Dis 9(10):326. https://doi.org/10.3390/jcdd9100326
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interest
Authors have no competing interest with any organization or person.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jain, P.K., Tadepalli, K.V., Roy, S. et al. Exploring deep learning for carotid artery plaque segmentation: atherosclerosis to cardiovascular risk biomarkers. Multimed Tools Appl 83, 42765–42797 (2024). https://doi.org/10.1007/s11042-023-17243-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11042-023-17243-3