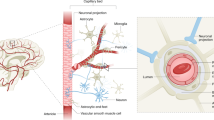
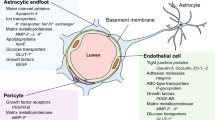
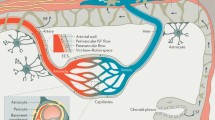
Abstract
Alzheimer’s disease (AD) is a prevalent neurodegenerative disorder characterized by progressive neuronal damage and cognitive decline. Recent studies have shed light on the involvement of not only the blood-brain barrier (BBB) dysfunction but also significant alterations in cellular junctions in AD pathogenesis. In this review article, we explore the role of the BBB and cellular junctions in AD pathology, with a specific focus on the hippocampus. The BBB acts as a crucial protective barrier between the bloodstream and the brain, maintaining brain homeostasis and regulating molecular transport. Preservation of BBB integrity relies on various junctions, including gap junctions formed by connexins, tight junctions composed of proteins such as claudins, occludin, and ZO-1, as well as adherence junctions involving molecules like vascular endothelial (VE) cadherin, Nectins, and Nectin-like molecules (Necls). Abnormalities in these junctions and junctional components contribute to impaired neuronal signaling and increased cerebrovascular permeability, which are closely associated with AD advancement. By elucidating the underlying molecular mechanisms governing BBB and cellular junction dysfunctions within the context of AD, this review offers valuable insights into the pathogenesis of AD and identifies potential therapeutic targets for intervention.

Similar content being viewed by others
Data availability
The datasets during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Jack CR Jr. et al (2018) NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement 14(4):535–562
Crous-Bou M et al (2017) Alzheimer’s disease prevention: from risk factors to early intervention. Alzheimers Res Ther 9(1):71
Podcasy JL, Epperson CN (2016) Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin Neurosci 18(4):437–446
Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019 Lancet Public Health, (2022) 7(2): p. e105-e125
Livingston G et al (2020) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396(10248):413–446
Li X et al (2022) Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2019. Front Aging Neurosci 14:937486
Calabrò M et al (2021) The biological pathways of Alzheimer disease: a review. AIMS Neurosci 8(1):86–132
Garcia MA, Nelson WJ, Chavez N (2018) Cell-cell junctions organize Structural and Signaling Networks, vol 10. Cold Spring Harb Perspect Biol, 4
Eftekhari A et al (2020) Cell junction proteins: crossing the glomerular filtration barrier in diabetic nephropathy. Int J Biol Macromol 148:475–482
Rao YL et al (2022) Hippocampus and its involvement in Alzheimer’s disease: a review. 3 Biotech 12(2):55
Machon O et al (2003) Role of beta-catenin in the developing cortical and hippocampal neuroepithelium. Neuroscience 122(1):129–143
Montagne A et al (2015) Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85(2):296–302
Nakase T, Naus CC (2004) Gap junctions and neurological disorders of the central nervous system. Biochim Biophys Acta 1662(1–2):149–158
Veeraval L, O’Leary CJ, Cooper HM (2020) Adherens junctions: guardians of cortical development. Front Cell Dev Biol 8:6
Ando K et al (2011) N-cadherin regulates p38 MAPK signaling via association with JNK-associated leucine zipper protein: implications for neurodegeneration in Alzheimer disease. J Biol Chem 286(9):7619–7628
Kocahan S, Doğan Z (2017) Mechanisms of Alzheimer’s Disease Pathogenesis and Prevention: the brain, neural Pathology, N-methyl-D-aspartate receptors, tau protein and other Risk factors. Clin Psychopharmacol Neurosci 15(1):1–8
Anwar MM et al (2022) Assessing the role of primary healthy microglia and gap junction blocker in hindering Alzheimer’s disease neuroinflammatory type: early approaches for therapeutic intervention. Front Neurosci 16:1041461
Kinney JW et al (2018) Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement (N Y) 4:575–590
Henstridge CM, Hyman BT, Spires-Jones TL (2019) Beyond the neuron-cellular interactions early in Alzheimer disease pathogenesis. Nat Rev Neurosci 20(2):94–108
Govindpani K et al (2019) Vascular Dysfunction in Alzheimer’s Disease: A Prelude to the Pathological Process or a Consequence of It?. J Clin Med, 8(5)
Koizumi K, Wang G, Park L (2016) Endothelial dysfunction and Amyloid-β-Induced neurovascular alterations. Cell Mol Neurobiol 36(2):155–165
Tamagno E et al (2021) Oxidative Stress and Beta Amyloid in Alzheimer’s Disease. Which Comes First: The Chicken or the Egg?. Antioxid (Basel), 10(9)
Sheng B et al (2009) Inhibition of gamma-secretase activity reduces abeta production, reduces oxidative stress, increases mitochondrial activity and leads to reduced vulnerability to apoptosis: implications for the treatment of Alzheimer’s disease. Free Radic Biol Med 46(10):1362–1375
Popov LD (2020) Mitochondrial biogenesis: an update. J Cell Mol Med 24(9):4892–4899
Golpich M et al (2017) Mitochondrial dysfunction and Biogenesis in neurodegenerative diseases: Pathogenesis and treatment. CNS Neurosci Ther 23(1):5–22
Sheng B et al (2012) Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer’s disease. J Neurochem 120(3):419–429
Reddy PH, Beal MF (2008) Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol Med 14(2):45–53
Zenaro E, Piacentino G, Constantin G (2017) The blood-brain barrier in Alzheimer’s disease. Neurobiol Dis 107:41–56
Ezan P et al (2012) Deletion of astroglial connexins weakens the blood-brain barrier. J Cereb Blood Flow Metab 32(8):1457–1467
Zhao Y et al (2018) Function of Connexins in the Interaction between glial and vascular cells in the Central Nervous System and related neurological diseases. Neural Plast 2018:p6323901
Cameron B, Landreth GE (2010) Inflammation, microglia, and Alzheimer’s disease. Neurobiol Dis 37(3):503–509
Wang WY et al (2015) Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med 3(10):136
Onyango IG et al (2021) Neuroinflammation in Alzheimer’s Disease. Biomedicines, 9(5)
Wyss-Coray T, Rogers J (2012) Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med 2(1):a006346
Goodenough DA, Paul DL (2009) Gap junctions. Cold Spring Harb Perspect Biol 1(1):a002576
Beyer EC, Berthoud VM (2018) Gap junction gene and protein families: connexins, innexins, and pannexins. Biochim Biophys Acta Biomembr 1860(1):5–8
Orellana JA, Martinez AD, Retamal MA (2013) Gap junction channels and hemichannels in the CNS: regulation by signaling molecules. Neuropharmacology 75:567–582
Elias LA, Kriegstein AR (2008) Gap junctions: multifaceted regulators of embryonic cortical development. Trends Neurosci 31(5):243–250
Chanson M et al (2005) Gap junctional communication in tissue inflammation and repair. Biochim Biophys Acta 1711(2):197–207
Dong A, Liu S, Li Y (2018) Gap junctions in the nervous system: probing functional connections using New Imaging approaches. Front Cell Neurosci 12:320
Dermietzel R et al (1989) Differential expression of three gap junction proteins in developing and mature brain tissues. Proc Natl Acad Sci U S A 86(24):10148–10152
Faber DS, Pereda AE (2018) Two forms of electrical transmission between neurons. Front Mol Neurosci 11:427
Wang Y, Pan Y, Li H (2020) What is brain health and why is it important? BMJ 371:m3683
Nichols MJ, Newsome WT (1999) The neurobiology of cognition. Nature 402(6761 Suppl):C35–C38
Hatch RJ et al (2017) Gap Junctions Link regular-spiking and fast-spiking interneurons in Layer 5 Somatosensory Cortex. Front Cell Neurosci 11:204
Traub RD et al (2001) Gap junctions between interneuron dendrites can enhance synchrony of gamma oscillations in distributed networks. J Neurosci 21(23):9478–9486
Pannasch U et al (2012) Astroglial gap junctions shape neuronal network activity. Commun Integr Biol 5(3):248–254
Crodelle J, McLaughlin DW (2021) Modeling the role of gap junctions between excitatory neurons in the developing visual cortex. PLoS Comput Biol 17(7):e1007915
Song D et al (2018) Chap. 7 - identification of neural plasticity from spikes. Handbook of behavioral neuroscience. Elsevier, pp 135–151. D. Manahan-Vaughan, Editor
Ghorbani M et al (2020) Impacts of epidural electrical stimulation on wnt signaling, FAAH, and BDNF following thoracic spinal cord injury in rat. J Cell Physiol 235(12):9795–9805
Hao L, Yang Z, Lei J (2018) Underlying mechanisms of Cooperativity, Input specificity, and associativity of long-term potentiation through a positive feedback of local protein synthesis. Front Comput Neurosci 12:25
Mesnil M et al (2020) Brain disorders and Chemical pollutants: a gap Junction Link?. Biomolecules, 11(1)
Li Q et al (2019) Targeting gap junction in epilepsy: perspectives and challenges. Biomed Pharmacother 109:57–65
Schwab BC et al (2014) Pallidal gap junctions-triggers of synchrony in Parkinson’s disease? Mov Disord 29(12):1486–1494
Markoullis K et al (2014) Oligodendrocyte gap junction loss and disconnection from reactive astrocytes in multiple sclerosis gray matter. J Neuropathol Exp Neurol 73(9):865–879
Belousov AB, Fontes JD (2013) Neuronal gap junctions: making and breaking connections during development and injury. Trends Neurosci 36(4):227–236
Itoh M, Bissell MJ (2003) The organization of tight junctions in epithelia: implications for mammary gland biology and breast tumorigenesis. J Mammary Gland Biol Neoplasia 8(4):449–462
Assimakopoulos SF, Papageorgiou I, Charonis A (2011) Enterocytes’ tight junctions: from molecules to diseases. World J Gastrointest Pathophysiol 2(6):123–137
Bhat AA et al (2018) Tight Junction proteins and Signaling pathways in Cancer and inflammation: a functional crosstalk. Front Physiol 9:p1942
Díaz-Coránguez M, Liu X, Antonetti DA (2019) Tight junctions in Cell Proliferation. Int J Mol Sci, 20(23)
Hartsock A, Nelson WJ (2008) Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta 1778(3):660–669
Takano K et al (2014) Role of tight junctions in signal transduction: an update. Excli j 13:1145–1162
Mandicourt G et al (2007) JAM-C regulates tight junctions and integrin-mediated cell adhesion and migration. J Biol Chem 282(3):1830–1837
Guan Z et al (2021) Blood-brain barrier, cell junctions, and Tumor Microenvironment in Brain metastases, the biological prospects and Dilemma in therapies. Front Cell Dev Biol 9:722917
Daneman R, Prat A (2015) The blood-brain barrier. Cold Spring Harb Perspect Biol 7(1):a020412
Kadry H, Noorani B, Cucullo L (2020) A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 17(1):69
Martins T et al (2011) Methamphetamine transiently increases the blood–brain barrier permeability in the hippocampus: role of tight junction proteins and matrix metalloproteinase-9. Brain Res 1411:28–40
Zhang X et al (2019) High-resolution mapping of brain vasculature and its impairment in the hippocampus of Alzheimer’s disease mice. Natl Sci Rev 6(6):1223–1238
Arthur FE, Shivers RR, Bowman PD (1987) Astrocyte-mediated induction of tight junctions in brain capillary endothelium: an efficient in vitro model. Brain Res 433(1):155–159
Greene C, Campbell M (2016) Tight junction modulation of the blood brain barrier: CNS delivery of small molecules. Tissue Barriers 4(1):e1138017
Lochhead JJ et al (2020) Structure, function, and regulation of the blood-brain barrier tight Junction in Central Nervous System disorders. Front Physiol 11:914
Meng W, Takeichi M (2009) Adherens junction: molecular architecture and regulation. Cold Spring Harb Perspect Biol 1(6):a002899
Gumbiner BM (2005) Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol 6(8):622–634
Mège RM, Ishiyama N (2017) Integration of cadherin adhesion and cytoskeleton at Adherens junctions. Cold Spring Harb Perspect Biol, 9(5)
Serra R, Simard JM (2023) Adherens, tight, and gap junctions in ependymal cells: a systematic review of their contribution to CSF-brain barrier. Front Neurol 14:1092205
Stamatovic SM et al (2016) Junctional proteins of the blood-brain barrier: new insights into function and dysfunction. Tissue Barriers 4(1):e1154641
Haddad-Tóvolli R et al (2017) Development and function of the blood-brain barrier in the Context of Metabolic Control. Front Neurosci 11:224
Stamatovic SM, Keep RF, Andjelkovic AV (2008) Brain endothelial cell-cell junctions: how to open the blood brain barrier. Curr Neuropharmacol 6(3):179–192
Montagne A, Zhao Z, Zlokovic BV (2017) Alzheimer’s disease: A matter of blood-brain barrier dysfunction? J Exp Med 214(11):3151–3169
Balasa R et al (2021) Reviewing the significance of blood-brain barrier disruption in multiple sclerosis Pathology and Treatment. Int J Mol Sci, 22(16)
Stocker AM, Chenn A (2015) The role of adherens junctions in the developing neocortex. Cell Adh Migr 9(3):167–174
Togashi H, Sakisaka T, Takai Y (2009) Cell adhesion molecules in the central nervous system. Cell Adh Migr 3(1):29–35
Yamashita M (2013) From neuroepithelial cells to neurons: changes in the physiological properties of neuroepithelial stem cells. Arch Biochem Biophys 534(1–2):64–70
Danglot L et al (2012) Vezatin is essential for dendritic spine morphogenesis and functional synaptic maturation. J Neurosci 32(26):9007–9022
Elia LP et al (2006) 120 catenin regulates dendritic spine and synapse development through rho-family GTPases and cadherins. Neuron 51(1):43–56
Arikkath J, Reichardt LF (2008) Cadherins and catenins at synapses: roles in synaptogenesis and synaptic plasticity. Trends Neurosci 31(9):487–494
Nikitczuk JS et al (2014) N-cadherin regulates molecular organization of excitatory and inhibitory synaptic circuits in adult hippocampus in vivo. Hippocampus 24(8):943–962
Heisler FF et al (2014) GRIP1 interlinks N-cadherin and AMPA receptors at vesicles to promote combined cargo transport into dendrites. Proc Natl Acad Sci U S A 111(13):5030–5035
Farías GG et al (2009) Wnt-5a/JNK signaling promotes the clustering of PSD-95 in hippocampal neurons. J Biol Chem 284(23):15857–15866
Dosemeci A et al (2007) Composition of the synaptic PSD-95 complex. Mol Cell Proteomics 6(10):1749–1760
Basu R, Taylor MR, Williams ME (2015) The classic cadherins in synaptic specificity. Cell Adh Migr 9(3):193–201
Guang S et al (2018) Synaptopathology involved in Autism Spectrum Disorder. Front Cell Neurosci 12:470
Forsyth JK, Lewis DA (2017) Mapping the consequences of impaired synaptic plasticity in Schizophrenia through Development: an integrative model for diverse clinical features. Trends Cogn Sci 21(10):760–778
Alahmari A (2021) Blood-brain barrier overview: structural and functional correlation. Neural Plast 2021:p6564585
Sharma C, Woo H, Kim SR (2022) Addressing Blood-Brain Barrier Impairment in Alzheimer’s Disease. Biomedicines, 10(4)
Rasmussen MK, Mestre H, Nedergaard M (2022) Fluid transport in the brain. Physiol Rev 102(2):1025–1151
Camandola S, Mattson MP (2017) Brain metabolism in health, aging, and neurodegeneration. Embo j 36(11):1474–1492
Dubey S et al (2020) Clinically approved IVIg delivered to the hippocampus with focused ultrasound promotes neurogenesis in a model of Alzheimer’s disease. Proc Natl Acad Sci U S A 117(51):32691–32700
Kempermann G, Song H, Gage FH (2015) Neurogenesis in the adult Hippocampus. Cold Spring Harb Perspect Biol 7(9):a018812
Solár P et al (2022) The blood-brain barrier and the neurovascular unit in subarachnoid hemorrhage: molecular events and potential treatments. Fluids Barriers CNS 19(1):29
Patabendige A, Janigro D (2023) The role of the blood-brain barrier during neurological disease and infection. Biochem Soc Trans 51(2):613–626
Sweeney MD, Sagare AP, Zlokovic BV (2018) Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 14(3):133–150
Tang QY et al (2021) Adiponectin mediates the Protection of H(2)S against Chronic Restraint stress-Induced Cognitive Impairment via attenuating hippocampal damage. Front Behav Neurosci 15:623644
Dunton AD et al (2021) Form and function of the Vertebrate and Invertebrate Blood-Brain barriers. Int J Mol Sci, 22(22)
Hladky SB, Barrand MA (2018) Elimination of substances from the brain parenchyma: efflux via perivascular pathways and via the blood-brain barrier. Fluids Barriers CNS 15(1):30
Pan YW, Storm DR, Xia Z (2013) Role of adult neurogenesis in hippocampus-dependent memory, contextual fear extinction and remote contextual memory: new insights from ERK5 MAP kinase. Neurobiol Learn Mem 105:81–92
Carvey PM, Hendey B, Monahan AJ (2009) The blood-brain barrier in neurodegenerative disease: a rhetorical perspective. J Neurochem 111(2):291–314
Nelson AR et al (2016) Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer’s disease. Biochim Biophys Acta 1862(5):887–900
Zlokovic BV (2008) The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57(2):178–201
Wang D et al (2021) Relationship between Amyloid-β deposition and blood-brain barrier dysfunction in Alzheimer’s Disease. Front Cell Neurosci 15:695479
Erickson MA, Banks WA (2013) Blood-brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J Cereb Blood Flow Metab 33(10):1500–1513
López-Ornelas A et al (2022) The Impairment of Blood-Brain Barrier in Alzheimer’s Disease: Challenges and Opportunities with Stem Cells. Int J Mol Sci, 23(17)
Lim YY et al (2018) Association of β-Amyloid and apolipoprotein E ε4 with memory decline in preclinical Alzheimer Disease. JAMA Neurol 75(4):488–494
Alata W et al (2015) Human apolipoprotein E ɛ4 expression impairs cerebral vascularization and blood-brain barrier function in mice. J Cereb Blood Flow Metab 35(1):86–94
Halliday MR et al (2016) Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J Cereb Blood Flow Metab 36(1):216–227
Bowman GL et al (2018) Blood-brain barrier breakdown, neuroinflammation, and cognitive decline in older adults. Alzheimers Dement 14(12):1640–1650
Janelidze S et al (2018) CSF biomarkers of neuroinflammation and cerebrovascular dysfunction in early Alzheimer disease. Neurology 91(9):e867–e877
Moon WJ et al (2021) Hippocampal blood-brain barrier permeability is related to the APOE4 mutation status of elderly individuals without dementia. J Cereb Blood Flow Metab 41(6):1351–1361
Ryu JK, McLarnon JG (2009) A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer’s disease brain. J Cell Mol Med 13(9a):2911–2925
Zenaro E et al (2015) Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med 21(8):880–886
Nation DA et al (2019) Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 25(2):270–276
Sengillo JD et al (2013) Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer’s disease. Brain Pathol 23(3):303–310
Jiang H et al (2011) Elevated CSF levels of TACE activity and soluble TNF receptors in subjects with mild cognitive impairment and patients with Alzheimer’s disease. Mol Neurodegener 6:69
Zhao Z et al (2015) Establishment and dysfunction of the blood-brain barrier. Cell 163(5):1064–1078
Rezai AR et al (2020) Noninvasive hippocampal blood-brain barrier opening in Alzheimer’s disease with focused ultrasound. Proc Natl Acad Sci U S A 117(17):9180–9182
Angeli S et al (2020) Altered expression of glial gap junction proteins Cx43, Cx30, and Cx47 in the 5XFAD model of Alzheimer’s disease. Front NeuroSci 14:582934
Nagy JI et al (1996) Elevated connexin43 immunoreactivity at sites of amyloid plaques in Alzheimer’s disease. Brain Res 717(1–2):173–178
O’Brien RJ, Wong PC (2011) Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci 34:185–204
Giaume C et al (2019) Connexins and pannexins in Alzheimer’s disease. Neurosci Lett 695:100–105
Kim Y et al (2017) Characterizing the mode of action of extracellular Connexin43 channel blocking mimetic peptides in an in vitro ischemia injury model. Biochim Biophys Acta Gen Subj 1861(2):68–78
Yi C et al (2016) Astroglial connexin43 contributes to neuronal suffering in a mouse model of Alzheimer’s disease. Cell Death Differ 23(10):1691–1701
Mei X et al (2010) Astroglial connexin immunoreactivity is specifically altered at β-amyloid plaques in β-amyloid precursor protein/presenilin1 mice. Neuroscience 171(1):92–105
Takeuchi H, Suzumura A (2014) Gap junctions and hemichannels composed of connexins: potential therapeutic targets for neurodegenerative diseases. Front Cell Neurosci 8:189
Maulik M et al (2020) Amyloid-β regulates gap junction protein connexin 43 trafficking in cultured primary astrocytes. J Biol Chem 295(44):15097–15111
Barres BA (2008) The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60(3):430–440
Koulakoff A et al (2012) Glial connexin expression and function in the context of Alzheimer’s disease. Biochim Biophys Acta 1818(8):2048–2057
Kunzelmann P et al (1999) Late onset and increasing expression of the gap junction protein connexin30 in adult murine brain and long-term cultured astrocytes. Glia 25(2):111–119
Kolchakova D et al (2021) Tight Junction protein Claudin-12 is involved in Cell Migration during Metastasis. Biomolecules, 11(5)
Zhu N et al (2022) Claudin-5 relieves cognitive decline in Alzheimer’s disease mice through suppression of inhibitory GABAergic neurotransmission. Aging 14(8):3554–3568
Lv J et al (2018) Focusing on claudin-5: a promising candidate in the regulation of BBB to treat ischemic stroke. Prog Neurobiol 161:79–96
Keaney J et al (2015) Autoregulated paracellular clearance of amyloid-β across the blood-brain barrier. Sci Adv 1(8):e1500472
Ueno M (2007) Molecular anatomy of the brain endothelial barrier: an overview of the distributional features. Curr Med Chem 14(11):1199–1206
Inagaki M et al (2003) Nectin-dependent localization of ZO-1 at puncta adhaerentia junctions between the mossy fiber terminals and the dendrites of the pyramidal cells in the CA3 area of adult mouse hippocampus. J Comp Neurol 460(4):514–524
Zhang H et al (2018) ZO-1 expression is suppressed by GM-CSF via miR-96/ERG in brain microvascular endothelial cells. J Cereb Blood Flow Metab 38(5):809–822
Romanitan MO et al (2007) Occludin is overexpressed in Alzheimer’s disease and vascular dementia. J Cell Mol Med 11(3):569–579
Cao Y et al (2015) Isoflurane anesthesia results in reversible ultrastructure and occludin tight junction protein expression changes in hippocampal blood-brain barrier in aged rats. Neurosci Lett 587:51–56
Wosik K et al (2007) Angiotensin II controls occludin function and is required for blood brain barrier maintenance: relevance to multiple sclerosis. J Neurosci 27(34):9032–9042
Takechi R et al (2008) Chylomicron amyloid-beta in the aetiology of Alzheimer’s disease. Atheroscler Suppl 9(2):19–25
Daneman R et al (2010) Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468(7323):562–566
Bell RD et al (2010) Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68(3):409–427
Costea L et al (2019) The blood-brain barrier and its intercellular junctions in Age-related Brain disorders. Int J Mol Sci, 20(21)
Liu CC et al (2020) Tau and apolipoprotein E modulate cerebrovascular tight junction integrity independent of cerebral amyloid angiopathy in Alzheimer’s disease. Alzheimers Dement 16(10):1372–1383
Milenkovic I, Petrov T, Kovacs GG (2014) Patterns of hippocampal tau pathology differentiate neurodegenerative dementias. Dement Geriatr Cogn Disord 38(5–6):375–388
Li Z et al (2020) APOE2: protective mechanism and therapeutic implications for Alzheimer’s disease. Mol Neurodegeneration 15(1):63
Ishii M, Iadecola C (2020) Risk factor for Alzheimer’s disease breaks the blood–brain barrier. Nature Publishing Group UK London
Brandon JA et al (2018) APOE and Alzheimer’s Disease: neuroimaging of metabolic and cerebrovascular dysfunction. Front Aging Neurosci 10:180
Tai LM et al (2016) The role of APOE in cerebrovascular dysfunction. Acta Neuropathol 131(5):709–723
Bell RD et al (2012) Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 485(7399):512–516
Yamazaki Y et al (2019) Selective loss of cortical endothelial tight junction proteins during Alzheimer’s disease progression. Brain 142(4):1077–1092
Marco S, Skaper SD (2006) Amyloid beta-peptide1-42 alters tight junction protein distribution and expression in brain microvessel endothelial cells. Neurosci Lett 401(3):219–224
Muradashvili N et al (2014) Ablation of MMP9 gene ameliorates paracellular permeability and fibrinogen-amyloid beta complex formation during hyperhomocysteinemia. J Cereb Blood Flow Metab 34(9):1472–1482
Giannotta M, Trani M, Dejana E (2013) VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell 26(5):441–454
Stefanova NA et al (2018) Association of cerebrovascular dysfunction with the development of Alzheimer’s disease-like pathology in OXYS rats. BMC Genomics 19(Suppl 3):75
Rosenberg GA (2009) Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol 8(2):205–216
Cheng T et al (2006) Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med 12(11):1278–1285
Zlokovic BV (2011) Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 12(12):723–738
Padden M et al (2007) Differences in expression of junctional adhesion molecule-A and beta-catenin in multiple sclerosis brain tissue: increasing evidence for the role of tight junction pathology. Acta Neuropathol 113(2):177–186
Wang Q et al (2022) Activation of Wnt/β-catenin pathway mitigates blood–brain barrier dysfunction in Alzheimer’s disease. Brain 145(12):4474–4488
Shimono Y et al (2012) Immunoglobulin superfamily receptors and adherens junctions. Subcell Biochem 60:137–170
Mizutani K et al (2021) Nectins and nectin-like molecules in synapse formation and involvement in neurological diseases. Mol Cell Neurosci 115:103653
Yang H et al (2013) Shotgun brain proteomics reveals early molecular signature in presymptomatic mouse model of Alzheimer’s disease. J Alzheimers Dis 37(2):297–308
Iturria-Medina Y et al (2016) Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun 7:11934
van de Haar HJ et al (2016) Blood-brain barrier leakage in patients with early Alzheimer Disease. Radiology 281(2):527–535
Jana A et al (2022) Increased type I interferon signaling and brain endothelial barrier dysfunction in an experimental model of Alzheimer’s disease. Sci Rep 12(1):16488
Hawkins BT, Davis TP (2005) The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57(2):173–185
Giri R et al (2000) beta-amyloid-induced migration of monocytes across human brain endothelial cells involves RAGE and PECAM-1. Am J Physiol Cell Physiol 279(6):C1772–C1781
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Keyvan Asghari and Zahra Niknam wrote the main manuscript text and Shadi Mohammadpour-Asl prepared the figure. Leyla Chodari wrote and modified the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have declared that there is no conflict of interest.
Financial interests
The authors declare they have no financial interests.
Declarations and statements
None of the authors are employed by a government agency. The only and primary function of all of the authors is research and all the authors have academic jobs. None of the authors are official representatives or on behalf of the government.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Asghari, K., Niknam, Z., Mohammadpour-Asl, S. et al. Cellular junction dynamics and Alzheimer’s disease: a comprehensive review. Mol Biol Rep 51, 273 (2024). https://doi.org/10.1007/s11033-024-09242-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09242-w