Abstract
Background
Trigeminal neuralgia (TN) is the most severe type of neuropathic pain. The trigeminal ganglion (TG) is a crucial target for the pathogenesis and treatment of TN. The colony-stimulating factor 1 (CSF1) - colony-stimulating factor 1 receptor (CSF1R) pathway regulates lower limb pain development. However, the effect and mechanism of the CSF1-CSF1R pathway in TG on TN are unclear.
Methods
Partial transection of the infraorbital nerve (pT-ION) model was used to generate a mouse TN model. Mechanical and cold allodynia were used to measure pain behaviors. Pro-inflammatory factors (IL-6, TNF-a) were used to measure inflammatory responses in TG. PLX3397, an inhibitor of CSF1R, was applied to inhibit the CSF1-CSF1R pathway in TG. This pathway was activated in naïve mice by stereotactic injection of CSF1 into the TG.
Results
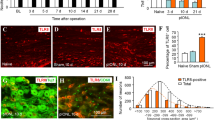
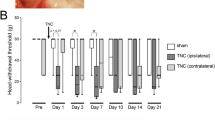
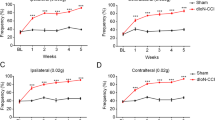
The TN model activated the CSF1-CSF1R pathway in the TG, leading to exacerbated mechanical and cold allodynia. TN activated inflammatory responses in the TG manifested as a significant increase in IL-6 and TNF-a levels. After using PLX3397 to inhibit CSF1R, CSF1R expression in the TG declined significantly. Inhibiting the CSF1-CSF1R pathway in the TG downregulated the expression of IL-6 and TNF-α to reduce allodynia-related behaviors. Finally, mechanical allodynia behaviors were exacerbated in naïve mice after activating the CSF1-CSF1R pathway in the TG.
Conclusions
The CSF1-CSF1R pathway in the TG modulates TN by regulating neuroimmune responses. Our findings provide a theoretical basis for the development of treatments for TN in the TG.





Similar content being viewed by others
Data availability
The datasets used in this study are available from the corresponding author upon reasonable request.
References
Cruccu G, Di Stefano G, Truini A (2020) Trigeminal neuralgia. N Engl J Med 383(8):754–762
Montano N, Conforti G, Di Bonaventura R et al (2015) Advances in diagnosis and treatment of trigeminal neuralgia. Ther Clin Risk Manag 11:289–299
Donertas-Ayaz B, Caudle RM (2023) Locus coeruleus-noradrenergic modulation of trigeminal pain: implications for trigeminal neuralgia and psychiatric comorbidities. Neurobiol Pain 13:100124
Holland M, Noeller J, Buatti J et al (2015) The cost-effectiveness of Surgery for trigeminal neuralgia in surgically naïve patients: a retrospective study. Clin Neurol Neurosurg 137:34–37
Lynds R, Lyu C, Lyu GW et al (2017) Neuronal plasticity of trigeminal ganglia in mice following nerve injury. J Pain Res 10:349–357
De Córdoba JL, García Bach M, Isach N et al (2015) Percutaneous Balloon Compression for Trigeminal Neuralgia: imaging and technical aspects. Reg Anesth Pain Med 40(5):616–622
Wu H, Zhou J, Chen J et al (2019) Therapeutic efficacy and safety of radiofrequency ablation for the treatment of trigeminal neuralgia: a systematic review and meta-analysis. J Pain Res 12:423–441
Patil S, Testarelli L (2021) Assessment of Growth factors, cytokines, and Cellular markers in saliva of patients with trigeminal Neuralgia. Molecules 26(10):2964
Martin SL, Reid AJ, Verkhratsky A et al (2019) Gene expression changes in dorsal root ganglia following peripheral nerve injury: roles in inflammation, cell death and nociception. Neural Regen Res 14(6):939–947
Zhu Y, Knolhoff BL, Meyer MA et al (2014) CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in Pancreatic cancer models. Cancer Res 74(18):5057–5069
Xu C, Zheng H, Liu T et al (2021) Bioinformatics analysis identifies CSF1R as an essential gene mediating neuropathic pain - experimental research. Int J Surg 95:106140
Kilkenny C, Browne WJ, Cuthill IC et al (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. J Pharmacol Pharmacother 1(2):94–99
Zhang SH, Yu J, Lou GD et al (2016) Widespread pain sensitization after partial infraorbital nerve transection in MRL/MPJ mice. Pain 157(3):740–749
Deuis JR, Dvorakova LS, Vetter I (2017) Methods used to Evaluate Pain behaviors in rodents. Front Mol Neurosci 10:284
Tang Y, Liu L, Xu D et al (2018) Interaction between astrocytic colony stimulating factor and its receptor on microglia mediates central sensitization and behavioral hypersensitivity in chronic post ischemic pain model. Brain Behav Immun 68:248–260
Whitehead JL, Ohara PT, Tauscher An et al (2003) A procedure to deliver herpes simplex virus to the murine trigeminal ganglion. Brain Res Brain Res Protoc 12(1):60–66
Cannarile MA, Weisser M, Jacob W et al (2017) Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer 5(1):53
Stanley ER, Chitu V (2014) CSF-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol 6(6):a021857
Thompson ML, Jimenez-Andrade JM, Chartier S et al (2015) Targeting cells of the myeloid lineage attenuates pain and Disease progression in a prostate model of Bone cancer. Pain 156(9):1692–1702
Wang GYF, Shi XQ, Wu W et al (2018) Sustained and repeated mouth opening leads to development of painful temporomandibular disorders involving macrophage/microglia activation in mice. Pain 159(7):1277–1288
Karai L, Brown DC, Mannes AJ et al (2004) Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest 113(9):1344–1352
Ju YY, Jiang M, Xu F et al (2021) CXCL10 and CXCR3 in the trigeminal ganglion contribute to Trigeminal Neuropathic Pain in mice. J Pain Res 14:41–51
Thomas Cheng H (2010) Spinal cord mechanisms of chronic pain and clinical implications. Curr Pain Headache Rep 14(3):213–220
Ji RR, Nackley A, Huh Y et al (2018) Neuroinflammation and Central Sensitization in chronic and widespread Pain. Anesthesiology 129(2):343–366
Clark AK, Old EA, Malcangio M (2013) Neuropathic pain and cytokines: current perspectives. J Pain Res 6:803–814
Acknowledgements
This study was supported by the National Key Research and Development Program of China (No.2018YFC2001905).
Funding
This study was supported by the National Key Research and Development Program of China (No.2018YFC2001905).
Author information
Authors and Affiliations
Contributions
Conceptualization: CX and YF; Methodology: ZH, CX and JG; Formal analysis and investigation: ZH, CX, JG, TL and YZ; Writing - original draft preparation: ZH and JG; Writing - review and editing: CX and YF; Funding acquisition: YF; Resources: YF; Supervision: YF. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Ethics Committee of Peking University People’s Hospital (2021PHE009) and followed the role of the care and use of laboratory animals.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, Z., Xu, C., Guo, J. et al. The CSF1-CSF1R pathway in the trigeminal ganglion mediates trigeminal neuralgia via inflammatory responses in mice. Mol Biol Rep 51, 215 (2024). https://doi.org/10.1007/s11033-023-09149-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-023-09149-y