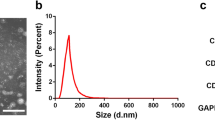
Abstract
Background
Spinal cord injury (SCI) due to lack of restoration of damaged neuronal cells is associated with sensorimotor impairment. This study was focused on using the human placental mesenchymal stem cells- exosome (HPMSCs- Exosomes) in an animal model of severe SCI under myelogram procedure.
Methods and results
Intrathecal injection of exosomes was performed in the acute phase of SCI in female rats. The improved functional recovery of the animals was followed for 6 weeks in control (saline, n = 6) and HPMSCs- EXO (HPMSCs-Exosomes, n = 6) groups. Pathological changes and glial scar size were evaluated. The Immunohistochemistry (IHC) of GFAP and NF200 factors as well as the apoptosis assay was investigated in the tissue samples from the injury site. The results demonstrated that HPMSCs-exosomes can improve motor function by attenuating apoptosis of neurons at the injury site, decreasing GFAP expression and increasing NF200 in the HPMSCs-EXO group. Also, HPMSCs-exosomes by preventing the formation of cavities causes preservation of tissue in SCI rats.
Conclusions
These findings demonstrate the effectiveness of HPMSC-Exosomes as a therapeutic method to improve functional recovery, reduce pathological changes associated with injury, and prevent chronicity after SCI. The neuroprotective and anti-apoptotic potential of HPMSCs- Exosomes may be a promising therapeutic approach for SCI. Another result was the importance of intrathecal injection of exosomes in the acute phase, which accelerated the healing process. Furthermore, the myelogram can be a feasible and suitable method to confirm the accuracy of intrathecal injection and examine the subarachnoid space in the laboratory animals.
Graphical abstract








Similar content being viewed by others
Data availability
All data would be available on request.
Abbreviations
- BBB:
-
Basso, beattie and bresnahan
- FBS:
-
Fetal bovine serum
- GFAP:
-
Glial fibrillary acidic protein
- H&E:
-
Hematoxylin–eosin
- hPMSCs:
-
Human mesenchymal stem cells
- hPMSCs-exosomes:
-
Human placental mesenchymal stem cells derived exosome
- IHC:
-
Immunohistochemistry
- LP:
-
Lumbar puncture
- MSC:
-
Mesenchymal stem cells
- NF200:
-
Neurofilament 200
- PFA:
-
Paraformaldehyde
- PBS:
-
Phosphate buffered saline
- SCI:
-
Spinal cord injury
- TEM:
-
Transmission electron microscopy
- TUNEL:
-
Terminal deoxynucleotidyl transferase-mediated dUTp nick end-labeling
References
David G, Mohammadi S, Martin AR et al (2019) Traumatic and nontraumatic spinal cord injury: pathological insights from neuroimaging. Nat Rev Neurol 15:718–731. https://doi.org/10.1038/s41582-019-0270-5
Hayta E, Elden H (2018) Acute spinal cord injury: a review of pathophysiology and potential of non-steroidal anti-inflammatory drugs for pharmacological intervention. J Chem Neuroanat 87:25–31. https://doi.org/10.1016/j.jchemneu.2017.08.001
Bradbury EJ, Burnside ER (2019) Moving beyond the glial scar for spinal cord repair. Nat Commun 10:3879. https://doi.org/10.1038/s41467-019-11707-7
Dalamagkas K, Tsintou M, Seifalian A, Seifalian AM (2018) Translational regenerative therapies for chronic spinal cord injury. Int J Mol Sci 19:1776. https://doi.org/10.3390/ijms19061776
Tashiro S, Tsuji O, Shinozaki M et al (2021) Current progress of rehabilitative strategies in stem cell therapy for spinal cord injury: a review. NPJ Regen Med 6:81. https://doi.org/10.1038/s41536-021-00191-7
Liau LL, Looi QH, Chia WC, Subramaniam T, Ng MH, Law JX (2020) Treatment of spinal cord injury with mesenchymal stem cells. Cell Biosci 10:1–17. https://doi.org/10.1186/s13578-020-00475-3
Ren Z, Qi Y, Sun S, Tao Y, Shi R (2020) Mesenchymal stem cell-derived exosomes: hope for spinal cord injury repair. Stem Cells Dev 29:1467–1478. https://doi.org/10.1089/scd.2020.0133
Kalluri R, LeBleu VS (2020) The biology, function, and biomedical applications of exosomes. Science 367:eaau6977. https://doi.org/10.1126/science.aau6977
He C, Zheng S, Luo Y, Wang B (2018) Exosome theranostics: biology and translational medicine. Theranostics 8:237. https://doi.org/10.7150/thno.21945
Yi H, Wang Y (2021) A meta-analysis of exosome in the treatment of spinal cord injury. Open Med 16:1043–1060. https://doi.org/10.1515/med-2021-0304
Maqsood M, Kang M, Wu X, Chen J, Teng L, Qiu L (2020) Adult mesenchymal stem cells and their exosomes: sources, characteristics, and application in regenerative medicine. Life Sci 256:118002. https://doi.org/10.1016/j.lfs.2020.118002
Sabapathy V, Herbert FJ, Kumar S (2017) Therapeutic application of placental mesenchymal stem cells reprogrammed neurospheres in spinal cord injury of SCID. Adult Stem Cells. https://doi.org/10.1007/978-1-4939-6756-8_8
Riau AK, Ong HS, Yam GH, Mehta JS (2019) Sustained delivery system for stem cell-derived exosomes. Front pharmacol 10:1368. https://doi.org/10.3389/fphar.2019.01368
Tator CH (2006) Review of treatment trials in humanspinal cord injury: issues, difficulties, and recommendations. Neurosurgery 59:957–987. https://doi.org/10.1227/01.neu.0000245591.16087.89
Bakshi A, Barshinger AL, Swanger SA et al (2006) Lumbar puncture delivery of bone marrow stromal cells in spinal cord contusion: a novel method for minimally invasive cell transplantation. J Neurotrauma 23:55–65. https://doi.org/10.1089/neu.2006.23.55
Bakshi A, Hunter C, Swanger S, Lepore A, Fischer I (2004) Minimally invasive delivery of stem cells for spinal cord injury: advantages of the lumbar puncture technique. J Neurosurg Spine 1:330–337. https://doi.org/10.3171/spi.2004.1.3.0330
Cizkova D, Murgoci A-N, Cubinkova V et al (2020) Spinal cord injury: animal models, imaging tools and the treatment strategies. Neurochem Res 45:134–143. https://doi.org/10.1007/s11064-019-02800-w
Yazdani SO, Pedram M, Hafizi M et al (2012) A comparison between neurally induced bone marrow derived mesenchymal stem cells and olfactory ensheathing glial cells to repair spinal cord injuries in rat. Tissue Cell 44:205–213. https://doi.org/10.1016/j.tice.2012.03.003
Sweis R, Biller J (2017) Systemic complications of spinal cord injury. Curr Neurol Neurosci Rep 17:1–8. https://doi.org/10.1007/s11910-017-0715-4
Cofano F, Boido M, Monticelli M et al (2019) Mesenchymal stem cells for spinal cord injury: current options, limitations, and future of cell therapy. Int J Mol Sci 20:2698. https://doi.org/10.3390/ijms20112698
Yeo RWY, Lai RC, Tan KH, Lim SK (2013) Exosome: a novel and safer therapeutic refinement of mesenchymal stem cell. J Circ Biomark 1:7. https://doi.org/10.5772/57460
Ha D, Yang N, Nadithe V (2016) Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B 6:287–296. https://doi.org/10.1016/j.apsb.2016.02.001
Nazarenko I, Rana S, Baumann A et al (2010) Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activationexosome-induced endothelial cell activation. Cancer Res 70:1668–1678. https://doi.org/10.1158/0008-5472.can-09-2470
Lu Y, Zhou Y, Zhang R et al (2019) Bone mesenchymal stem cell-derived extracellular vesicles promote recovery following spinal cord injury via improvement of the integrity of the blood-spinal cord barrier. Front Neurosci 13:209. https://doi.org/10.3389/fnins.2019.00209
Zhao C, Zhou X, Qiu J et al (2019) Exosomes derived from bone marrow mesenchymal stem cells inhibit complement activation in rats with spinal cord injury. Drug Des Devel Ther. https://doi.org/10.2147/DDDT.S209636
Liang Y, Wu J-H, Zhu J-H, Yang H (2022) Exosomes secreted by hypoxia–pre-conditioned adipose-derived mesenchymal stem cells reduce neuronal apoptosis in rats with spinal cord injury. J Neurotrauma 39:701–714. https://doi.org/10.1089/neu.2021.0290
Sun G, Li G, Li D et al (2018) hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater Sci Eng C 89:194–204. https://doi.org/10.1016/j.msec.2018.04.006
Zhong D, Cao Y, Li C-J et al (2020) Neural stem cell-derived exosomes facilitate spinal cord functional recovery after injury by promoting angiogenesis. Exp Biol Med 245:54–65. https://doi.org/10.1177/1535370219895491
Yuan X, Wu Q, Wang P et al (2019) Exosomes derived from pericytes improve microcirculation and protect blood–spinal cord barrier after spinal cord injury in mice. Front Neurosci 13:319. https://doi.org/10.3389/fnins.2019.00319
Zhang B, Lin F, Dong J, Liu J, Ding Z, Xu J (2021) Peripheral macrophage-derived exosomes promote repair after spinal cord injury by inducing local anti-inflammatory type microglial polarization via increasing autophagy. Int J Biol Sci 17:1339. https://doi.org/10.7150/ijbs.54302
Cao Y, Xu Y, Chen C, Xie H, Lu H, Hu J (2021) Local delivery of USC-derived exosomes harboring ANGPTL3 enhances spinal cord functional recovery after injury by promoting angiogenesis. J Stem Cell Res Ther 12:1–17. https://doi.org/10.1186/s13287-020-02078-8
Peng W, Wan L, Luo Z et al (2021) Microglia-derived exosomes improve spinal cord functional recovery after injury via inhibiting oxidative stress and promoting the survival and function of endothelia cells. Oxid Med Cell Longev. https://doi.org/10.1155/2021/1695087
Huang J-H, Xu Y, Yin X-M, Lin F-Y (2020) Exosomes derived from miR-126-modified MSCs promote angiogenesis and neurogenesis and attenuate apoptosis after spinal cord injury in rats. Neuroscience 424:133–145. https://doi.org/10.1016/j.neuroscience.2019.10.043
Kulubya ES, Clark K, Hao D et al (2021) The unique properties of placental mesenchymal stromal cells: a novel source of therapy for congenital and acquired spinal cord injury. Cells 10:2837. https://doi.org/10.3390/cells10112837
Deng J, Li M, Meng F et al (2021) 3D spheroids of human placenta-derived mesenchymal stem cells attenuate spinal cord injury in mice. J Cell Death 12:1096. https://doi.org/10.1038/s41419-021-04398-w
Zhang C, Zhang C, Xu Y, Li C, Cao Y, Li PJNL (2020) Exosomes derived from human placenta-derived mesenchymal stem cells improve neurologic function by promoting angiogenesis after spinal cord injury. Neurosci Lett 739:135399. https://doi.org/10.1016/j.neulet.2020.135399
Zhou W, Silva M, Feng C et al (2021) Exosomes derived from human placental mesenchymal stem cells enhanced the recovery of spinal cord injury by activating endogenous neurogenesis. Stem Cell Res Ther 12:1–14. https://doi.org/10.1186/s13287-021-02248-2
Lee J-R, Kyung JW, Kumar H et al (2020) Targeted delivery of mesenchymal stem cell-derived nanovesicles for spinal cord injury treatment. Int J Mol Sci 21:4185. https://doi.org/10.3390/ijms21114185
Janssen M, Nabih A, Moussa W, Kawchuk GN, Carey JP (2011) Evaluation of diagnosis techniques used for spinal injury related back pain. Pain Res Treat. https://doi.org/10.1155/2011/478798
Ahmed RU, Alam M, Zheng Y-P (2019) Experimental spinal cord injury and behavioral tests in laboratory rats. Heliyon 5:e01324. https://doi.org/10.1016/j.heliyon.2019.e01324
Courtine G, Sofroniew MV (2019) Spinal cord repair: advances in biology and technology. Nat Med 25:898–908. https://doi.org/10.1038/s41591-019-0475-6
Lapuente-Chala C, Céspedes-Rubio A (2018) Biochemical events related to glial response in spinal cord injury. Rev Fac Med 66:269–277. https://doi.org/10.15446/revfacmed.v66n2.61701
Liu W, Wang Y, Gong F et al (2019) Exosomes derived from bone mesenchymal stem cells repair traumatic spinal cord injury by suppressing the activation of A1 neurotoxic reactive astrocytes. J Neurotrauma 36:469–484. https://doi.org/10.1089/neu.2018.5835
Jia Y, Lu T, Chen Q et al (2021) Exosomes secreted from sonic hedgehog-modified bone mesenchymal stem cells facilitate the repair of rat spinal cord injuries. Acta Neurochir 163:2297–2306. https://doi.org/10.1007/s00701-021-04829-9
He Y, Liu X, Chen Z (2020) Glial scar—a promising target for improving outcomes after CNS injury. J Mol Neurosci 70:340–352. https://doi.org/10.1007/s12031-019-01417-6
Xie C, Shen X, Xu X et al (2020) Astrocytic YAP promotes the formation of glia scars and neural regeneration after spinal cord injury. J Neurosci 40:2644–2662. https://doi.org/10.1523/JNEUROSCI.2229-19.2020
Brenner M (2014) Role of GFAP in CNS injuries. Neurosci Lett 565:7–13. https://doi.org/10.1016/j.neulet.2014.01.055
Huang W, Lin M, Yang C et al (2021) Rat bone mesenchymal stem cell-derived exosomes loaded with miR-494 promoting neurofilament regeneration and behavioral function recovery after spinal cord injury. Oxid Med Cell Longev. https://doi.org/10.1155/2021/1634917
Xiao X, Li W, Rong D et al (2021) Human umbilical cord mesenchymal stem cells-derived extracellular vesicles facilitate the repair of spinal cord injury via the miR-29b-3p/PTEN/Akt/mTOR axis. Cell Death Discov 7:212. https://doi.org/10.1038/s41420-021-00572-3
Yu T, Zhao C, Hou S, Zhou W, Wang B, Chen Y (2019) Exosomes secreted from miRNA-29b-modified mesenchymal stem cells repaired spinal cord injury in rats. Braz J Med Biol Res. https://doi.org/10.1590/1414-431X20198735
Acknowledgements
We are very grateful to everyone who contributed to the success of this research project.
Funding
This study was supported by the Clinical Biochemistry department of Tarbiat Modares University, Medical Sciences.
Author information
Authors and Affiliations
Contributions
AS: performed the experiments, analyzed data, and wrote the manuscript. SOY: discussed the results and analysis the data. MP: designed and made the animal model and the myelogram. FS: was a surgical assistant to create the animal model. MJR: designed research, discussed the results, edited this manuscript, and provided technical and material support. MS: designed the project and performed the development of methodology. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Ethical approval
All animal operations were performed in accordance with the laboratory animal standards set by the Animal Ethics Committee of the Tarbiat Modares University of Medical Science (code of ethics: IR.MODARES.REC.1397.064).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soleimani, A., Oraee Yazdani, S., Pedram, M. et al. Intrathecal injection of human placental mesenchymal stem cells derived exosomes significantly improves functional recovery in spinal cord injured rats. Mol Biol Rep 51, 193 (2024). https://doi.org/10.1007/s11033-023-08972-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-023-08972-7