Abstract
Background
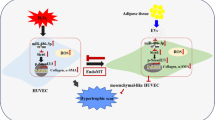
Human mesenchymal stem/stromal cells (hMSCs) are known for their pronounced therapeutic potential; however, they are still applied in limited clinical cases for several reasons. ROS-mediated oxidative stress is among the chief causes of post-transplantation apoptosis and death of hMSCs. It has been reported that a strategy to protect hMSCs against ROS is to pretreat them with antioxidants. Oleoylethanolamide (OEA) is a monounsaturated fatty acid derived from oleic acid and it has many protective properties, including anti-obesity, anti-inflammatory, and antioxidant effects. OEA is also used as a weight loss supplement; due to its high affinity for the PPAR-α receptor, OEA increases the fat metabolism rate.
Methods and results
This study hence assessed the effects of OEA pretreatment on the in vitro survival rate and resistance of hMSCs under oxidative stress as well as the cellular and molecular events in the biology of stem/stromal cells affected by oxidative stress and free radicals. Considering the role of MSCs in adipogenesis and obesity, the expression of the main genes involved in adipogenesis was also addressed in this study. Results revealed that OEA increases the in vitro proliferation of MSCs and inhibits cell apoptosis by reducing the induction of oxidative stress. The results also indicated that OEA exerts its antioxidant properties by both activating the Nrf2/NQO-1/HO-1 signaling pathway and directly combating free radicals. Moreover, OEA can reduce adipogenesis through reducing the expression of PPARγ, leptin and CEBPA genes in hMSCs undergoing adipocyte differentiation.
Conclusions
Thus, OEA protects hMSCs from oxidative stress and reduces adipogenic related genes expression and can be regarded as a therapeutic agent for this purpose.






Similar content being viewed by others
Data availability
No data associated in the manuscript.
References
Markov A et al (2021) Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res Ther 12(1):1–30
Aprile D et al (2021) MUSE stem cells can be isolated from stromal compartment of mouse bone marrow, adipose tissue, and ear connective tissue: a comparative study of their in vitro properties. Cells 10(4):761
Brown C et al (2019) Mesenchymal stem cells: cell therapy and regeneration potential. J Tissue Eng Regen Med 13(9):1738–1755
Margiana R et al (2022) Clinical application of mesenchymal stem cell in regenerative medicine: a narrative review. Stem Cell Res Ther 13(1):1–22
Zhou T et al (2021) Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol 14(1):1–24
Barzegari A et al (2020) Mitochondria-targeted antioxidant mito‐TEMPO alleviate oxidative stress induced by antimycin A in human mesenchymal stem cells. J Cell Physiol 235(7–8):5628–5636
Barzegari A et al (2020) The role of Hippo signaling pathway and mechanotransduction in tuning embryoid body formation and differentiation. J Cell Physiol 235(6):5072–5083
Valverde M et al (2018) Hydrogen peroxide-induced DNA damage and repair through the differentiation of human adipose-derived mesenchymal stem cells. Stem Cells Int. https://doi.org/10.1155/2018/1615497
Amiri F, Jahanian-Najafabadi A, Roudkenar MH (2015) Vitro augmentation of mesenchymal stem cells viability in stressful microenvironments: in vitro augmentation of mesenchymal stem cells viability. Cell Stress Chaperones 20:237–251
Barzegari A et al (2022) The protective effect of N-acetylcysteine on antimycin A-induced respiratory chain deficiency in mesenchymal stem cells. Chemico-Biol Interact 360:109937
Zeng W et al (2015) Antioxidant treatment enhances human mesenchymal stem cell anti-stress ability and therapeutic efficacy in an acute liver failure model. Sci Rep 5(1):1–17
Mias C et al (2008) Ex vivo pretreatment with melatonin improves survival, proangiogenic/mitogenic activity, and efficiency of mesenchymal stem cells injected into ischemic kidney. Stem Cells 26(7):1749–1757
Romano A et al (2021) Chronic oleoylethanolamide treatment decreases hepatic triacylglycerol level in rat liver by a PPARγ/SREBP-mediated suppression of fatty acid and triacylglycerol synthesis. Nutrients 13(2):394
Piomelli D (2013) A fatty gut feeling. Trends Endocrinol Metab 24(7):332–341
Bowen KJ et al (2017) Oleic acid-derived oleoylethanolamide: a nutritional science perspective. Prog Lipid Res 67:1–15
Giudetti AM et al (2021) Oleoylethanolamide reduces hepatic oxidative stress and endoplasmic reticulum stress in high-fat diet-fed rats. Antioxidants 10(8):1289
Hu J et al (2021) Oleoylethanolamide protects against acute liver injury by regulating Nrf-2/HO-1 and NLRP3 pathways in mice. Front Pharmacol 11:605065
Guzmán M et al (2004) Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor α (PPAR-α). J Biol Chem 279(27):27849–27854
Wang X, Miyares RL, Ahern GP (2005) Oleoylethanolamide excites vagal sensory neurones, induces visceral pain and reduces short-term food intake in mice via capsaicin receptor TRPV1. J Physiol 564(2):541–547
Manna P, Jain SK (2015) Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metab Syndr Relat Disord 13(10):423–444
Jo J et al (2009) Hypertrophy and/or hyperplasia: dynamics of adipose tissue growth. PLoS Comput Biol 5(3):e1000324
Jakab J et al (2021) Adipogenesis as a potential anti-obesity target: a review of pharmacological treatment and natural products. Diabetes, Metab Syndrome Obes. https://doi.org/10.2147/DMSO.S281186
Moreno-Navarrete JM, Fernández-Real JM (2017) Adipocyte differentiation. In: Symonds M (ed) Adipose tissue biology. Springer, Cham, pp 60–90
Li D et al (2013) Mesenchymal stem cells protect podocytes from apoptosis induced by high glucose via secretion of epithelial growth factor. Stem Cell Res Ther 4(5):1–11
Mohammadi S et al (2021) Astaxanthin protects mesenchymal stem cells from oxidative stress by direct scavenging of free radicals and modulation of cell signaling. Chemico-Biol Interact 333:109324
Forman HJ, Davies KJ, Ursini F (2014) How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic Biol Med 66:24–35
Furfaro A et al (2016) The Nrf2/HO-1 axis in cancer cell growth and chemoresistance. Oxid Med Cell Longev https://doi.org/10.1155/2016/1958174
Esmaeili MA et al (2016) Viola plant cyclotide vigno 5 induces mitochondria-mediated apoptosis via cytochrome C release and caspases activation in cervical cancer cells. Fitoterapia 109:162–168
Su S-H et al (2013) Caffeine inhibits adipogenic differentiation of primary adipose-derived stem cells and bone marrow stromal cells. Toxicol in Vitro 27(6):1830–1837
Zhang W et al (2009) Berberine protects mesenchymal stem cells against hypoxia-induced apoptosis in vitro. Biol Pharm Bull 32(8):1335–1342
Pouryousefi E et al (2022) Improved glycemic status, insulin resistance and inflammation after receiving oral oleoylethanolamide supplement in people with prediabetes: a randomized controlled trial. Diabetol Metab Syndr 14(1):1–9
Dairaku N et al (2004) Oligomycin and antimycin A prevent nitric oxide-induced apoptosis by blocking cytochrome C leakage. J Lab Clin Med 143(3):143–151
Ogita M et al (2009) Antimycin A-induced cell death depends on AIF translocation through NO production and PARP activation and is not involved in ROS generation, cytochrome c release and caspase-3 activation in HL-60 cells. J Antibiot 62(3):145–152
Chan JZ et al (2022) N-oleoylethanolamide treatment of lymphoblasts deficient in tafazzin improves cell growth and mitochondrial morphology and dynamics. Sci Rep 12(1):1–15
Niki E (2010) Assessment of antioxidant capacity in vitro and in vivo. Free Radic Biol Med 49(4):503–515
Hu SSJ, Mackie K (2015) Distribution of the endocannabinoid system in the central nervous system, in endocannabinoids. Springer, Berlin, pp 59–93
Gulaya NM et al (1998) Long-chain N-acylethanolamines inhibit lipid peroxidation in rat liver mitochondria under acute hypoxic hypoxia. Chem Phys Lipids 97(1):49–54
Di Marzo N, Chisci E, Giovannoni R (2018) The role of hydrogen peroxide in redox-dependent signaling: homeostatic and pathological responses in mammalian cells. Cells 7(10):156
Chen H-Y et al (2012) The protective effect of 17β-estradiol against hydrogen peroxide-induced apoptosis on mesenchymal stem cell. Biomed Pharmacother 66(1):57–63
Kim JY et al (2015) Pretreatment with lycopene attenuates oxidative stress-induced apoptosis in human mesenchymal stem cells. Biomol Ther 23(6):517
Ma Q (2013) Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53:401
Raghunath A et al (2018) Antioxidant response elements: discovery, classes, regulation and potential applications. Redox Biol 17:297–314
Chiang S-K, Chen S-E, Chang L-C (2021) The role of HO-1 and its crosstalk with oxidative stress in cancer cell survival. Cells 10(9):2401
Dinkova-Kostova AT, Talalay P (2010) NAD (P) H: quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys 501(1):116–123
Purdom-Dickinson SE et al (2007) Induction of antioxidant and detoxification response by oxidants in cardiomyocytes: evidence from gene expression profiling and activation of Nrf2 transcription factor. J Mol Cell Cardiol 42(1):159–176
Zorova LD et al (2018) Mitochondrial membrane potential. Anal Biochem 552:50–59
Owusu-Ansah E, Banerjee U (2009) Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature 461(7263):537–541
Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24(10):R453–R462
Acknowledgements
The authors like to acknowledge the financial and technical support provided by the Dietary Supplements & Probiotic Research Center at Alborz University of Medical Sciences. The authors also like to acknowledge Dr. abolfazl barzegari for his technical assistant for this study.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
SE and FZ developed the idea. SE acquired funding, conceived and directed the project. FZ set up the study, carried out cellular and molecular tests with contributions from AS, SP, HM and AT. FZ prepared the first draft of the article, and completed writing with contributions from SG-F. SE revised and completed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
The study protocol was approved by the ethical committee of Alborz University of Medical Sciences (IR.ABZUMS.REC.1400.200). All methods were performed in accordance with the relevant guidelines and regulations.
Consent to participant
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zare, F., Ghafouri-Fard, S., Shamosi, A. et al. Oleoylethanolamide protects mesenchymal stem/stromal cells (MSCs) from oxidative stress and reduces adipogenic related genes expression in adipose-derived MSCs undergoing adipocyte differentiation. Mol Biol Rep 51, 33 (2024). https://doi.org/10.1007/s11033-023-08929-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-023-08929-w