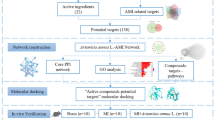
Abstract
Objectives of the Study
The aim of this study is to investigate whether fisetin can effectively reduce the myocardial damage induced by patulin. This study also aims to reveal the mechanism and target of fisetin in inhibiting myocardial damage.
Materials and Methods
Network pharmacology was used to screen the targets of fisetin on myocardial damage and the regulatory network of active ingredients-drug targets was constructed. GO and KEGG enrichment analyses were performed to screen out the key pathways and targets of fisetin on myocardial damage. Patulin induced apoptosis in H9c2 cardiomyocytes to verify the key targets. The mechanism of fisetin in inhibiting myocardial damage was determined.
Results
FIS can reduce the apoptosis of cardiomyocytes by protecting cardiomyocytes from PAT injury. According to the results of network pharmacology analysis, combined with enzyme activity detection and WB experiment, it was found that the mechanism of FIS to reduce myocardial damage may be related to the P53 signaling pathway, Caspase3/8/9 and Bax/Bcl-2.
Conclusion
FIS plays a protective role in PAT-induced myocardial damage. On the one hand, FIS inhibits the protein overexpression of P53, Caspase-9 and Bax. On the other hand, FIS enhances the protein expression of Bcl-2.





Similar content being viewed by others
References
Al-Hazmi MA (2014) Patulin in apple juice and its risk assessments on albino mice. Toxicol Ind Health 30(6):534–545
Xing M, Chen Y, Li B, Tian S (2021) “Characterization of a short-chain dehydrogenase/reductase and its function in patulin biodegradation in apple juice,“ Food Chemistry, vol. 348, p. 129046, /06/30/ 2021
Schumacher DM, Müller C, Metzler M, Lehmann L (2006) “DNA–DNA cross-links contribute to the mutagenic potential of the mycotoxin patulin,“ Toxicology Letters, vol. 166, no. 3, pp. 268–275, /10/25/ 2006
Zhang B, Peng X, Li G, Xu Y, Xia X, Wang Q (2015) “Oxidative stress is involved in Patulin induced apoptosis in HEK293 cells,“ Toxicon, vol. 94, pp. 1–7, 2015/02/01/
Zhang B, Huang C, Lu Q, Liang H, Li J, Xu D (2022) “Involvement of caspase in patulin-induced hepatotoxicity in vitro and in vivo,“ Toxicon, vol. 206, pp. 64–73, 2022/01/30/
Puel O, Galtier P, Oswald IP “Biosynthesis and Toxicological Effects of Patulin,“ Toxins, vol. 2, no. 4, pp. 613–631. doi: https://doi.org/10.3390/toxins2040613
Alam S, Pal A, Kumar R, Dwivedi PD, Das M, Ansari KM “EGFR-mediated akt and MAPKs signal pathways play a crucial role in patulin-induced cell proliferation in primary murine keratinocytes via modulation of cyclin D1 and COX-2 expression,“Molecular Carcinogenesis, vol. 53, no. 12, pp.988–998, 2014/12/01 2014.
Assunção R, Pinhão M, Loureiro S, Alvito P, Silva MJ (2019) “A multi-endpoint approach to the combined toxic effects of patulin and ochratoxin a in human intestinal cells,“ Toxicology Letters, vol. 313, pp. 120–129, /10/01/ 2019
Zhang B et al (2022) “Cardiotoxicity of patulin was found in H9c2 cells,“ Toxicon, vol. 207, pp. 21–30, 2022/02/01/
Sundarraj K, Raghunath A, Perumal E (2018) A review on the chemotherapeutic potential of fisetin: in vitro evidences. Biomed Pharmacother 97:928–940 2018/01/01/
Khan N, Syed DN, Ahmad N, Mukhtar H “Fisetin: a dietary antioxidant for Health Promotion,“Antioxidants & Redox Signaling, vol. 19, no. 2, pp.151–162, 2013/07/10 2012.
Sivakumar B, Boovarahan SR, Prem PN, Kurian GA (2021) “Fisetin ameliorates ischemia re-oxygenation injury in H9c2 cardiomyocytes via targeting the PI3K signalling pathway,“ Phytomedicine Plus, vol. 1, no. 3, p. 100094, /08/01/ 2021
Altamimi JZ, BinMowyna MN, AlFaris NA, Alagal RI, El-kott AF, Al-Farga AM (2021) “Fisetin protects against streptozotocin-induced diabetic cardiomyopathy in rats by suppressing fatty acid oxidation and inhibiting protein kinase R,“ Saudi Pharmaceutical Journal, vol. 29, no. 1, pp. 27–42, /01/01/ 2021
Althunibat OY, Al Hroob AM, Abukhalil MH, Germoush MO, Bin-Jumah M, Mahmoud AM (2019) “Fisetin ameliorates oxidative stress, inflammation and apoptosis in diabetic cardiomyopathy,“ Life Sciences, vol. 221, pp. 83–92, /03/15/ 2019
Brand B et al “Identification of Patulin from Penicillium coprobium as a Toxin for Enteric Neurons,“ Molecules, vol. 24, no. 15. doi: https://doi.org/10.3390/molecules24152776
Hsu S-S, Lin Y-S, Chio L-M, Liang W-Z (2023) “Evaluation of the mycotoxin patulin on cytotoxicity and oxidative stress in human glioblastoma cells and investigation of protective effect of the antioxidant N-acetylcysteine (NAC),“ Toxicon, vol. 221, p. 106957, /01/01/ 2023
Chen H et al (2022) “Patulin disrupts SLC7A11-cystine-cysteine-GSH antioxidant system and promotes renal cell ferroptosis both in vitro and in vivo,“ Food and Chemical Toxicology, vol. 166, p. 113255, /08/01/ 2022
Ma T, Kandhare AD, Mukherjee-Kandhare AA, Bodhankar SL “Fisetin, a plant flavonoid ameliorates doxorubicin-induced cardiotoxicity in experimental rats: the decisive role of caspase-3, COX-II, cTn-I, iNOs and TNF-α,“Molecular Biology Reports, vol. 46, no. 1, pp.105–118, 2019/02/01 2019.
Wyllie AH “Apoptosis and the regulation of cell numbers in normal and neoplastic tissues: an overview,“Cancer and Metastasis Reviews, vol. 11, no. 2, pp.95–103, 1992/09/01 1992.
Jin Z, El-Deiry WS “Overview of cell death signaling pathways,“Cancer Biology & Therapy, vol. 4, no. 2, pp.147–171, 2005/02/02 2005.
Green DR, Knight RA, Melino G, Finazzi-Agro A, Orrenius S “Ten years of publication in cell death,“Cell Death & Differentiation, vol. 11, no. 1, pp.2–3, 2004/01/01 2004.
Adams JM, Cory S “The Bcl-2 protein family: arbiters of cell survival,“Science, vol. 281, no. 5381, pp.1322–1326, 1998/08/28 1998.
Ofengeim D, Miyawaki T, Suzanne zukin R (2011) “6 - Molecular and Cellular Mechanisms of Ischemia-Induced Neuronal Death,“ in Stroke (Fifth Edition), J. P. Mohr, P. A. Wolf, J. C. Grotta, M. A. Moskowitz, M. R. Mayberg, and R. von Kummer, Eds. Saint Louis: W.B. Saunders, pp. 75–106
Tarhan G (2019) “Membrane receptors: the Basic structure and functions,“Advances in Complementary & Alternative medicine, vol. 5, no. 3,
Fadeel B, Ottosson A, Pervaiz S “Big wheel keeps on turning: apoptosome regulation and its role in chemoresistance,“Cell Death & Differentiation, vol. 15, no. 3, pp.443–452, 2008/03/01 2008.
Zhu Y et al “The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs,“Aging Cell, vol. 14, no. 4, pp.644–658, 2015/08/01 2015.
Zhu DE, Pirtskhalava Y, Giorgadze T, Wentworth N, Fuhrmann-Stroissnigg M, Niedernhofer H, Robbins LJ, Tchkonia PD, Kirkland T (2017) New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging (Albany NY) vol 9(3):955–963
Yousefzadeh MJ et al (2018) “Fisetin is a senotherapeutic that extends health and lifespan,“ EBioMedicine, vol. 36, pp. 18–28, /10/01/ 2018
Singh S, Singh AK, Garg G, Rizvi SI (2018) Fisetin as a caloric restriction mimetic protects rat brain against aging induced oxidative stress, apoptosis and neurodegeneration. Life Sci 193:171–179 2018/01/15/
Singh S, Garg G, Singh AK, Tripathi SS, Rizvi SI “Fisetin, a potential caloric restriction mimetic, modulates ionic homeostasis in senescence induced and naturally aged rats,“Archives of Physiology and Biochemistry, vol. 128, no. 1, pp.51–58, 2022/01/02 2022.
Tang C et al (2019) “P53 in kidney injury and repair: Mechanism and therapeutic potentials,“ Pharmacology & Therapeutics, vol. 195, pp. 5–12, /03/01/ 2019
Nagata M (2016) Podocyte injury and its consequences. Kidney Int 89(6):1221–1230 2016/06/01/
Shen Y, White E (2001) “p53-Dependent apoptosis pathways,“ in Advances in Cancer Research, vol. 82: Academic Press, pp. 55–84
Chi SW (2014) “Structural insights into the transcription-independent apoptotic pathway of p53,“ BMB Rep, vol. 47, no. 3, pp. 167 – 72, Mar
Ackonwledgements
This work was supported by the National Natural Science Foundation of China (31,760,495); the Nature Fund of Gansu Province(18JR3RA136); Innovation Star Project of Excellent Graduate Students in Gansu Province(2022CZCZ-494).
Funding
This work was supported by the National Natural Science Foundation of China (31760495); the Nature Fund of Gansu Province(18JR3RA136); Innovation Star Project of Excellent Graduate Students in Gansu Province(2022CZCZ-494).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Baigang Zhang: Determine the subject, Formal analysis, Investigation, Methodology, Validation, Visualization, Funding support. Dongmei Xu: Determine the subject, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing-review & editing. Chenghui Huang: Determine the subject, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing-review & editing. Lu Jiao: Formal analysis, Investigation, Methodology, Software, Investigation, Methodology, Validation, Writing-original draft, Writing - review & editing. Yang Li: Methodology, Visualization, Formal analysis, Software, Investigation, Project administration. Binggang Fu: Formal analysis, investigation, visualization, software, verification. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dongmei Xu and Baigang Zhang these authors contributed equally to this work.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, D., Zhang, B., Huang, C. et al. Effect and mechanism of Fisetin on myocardial damage induced by Patulin. Mol Biol Rep 50, 6579–6589 (2023). https://doi.org/10.1007/s11033-023-08379-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08379-4