Abstract
Background
Chondrocytes are the only cell components in the cartilage, which has the poor regeneration ability. Thus, repairing damaged cartilage remains a huge challenge. Sika deer antlers are mainly composed of cartilaginous tissues that have an astonishing capacity for repair and renewal. Our previous study has demonstrated the transforming growth factor β (TGF-β1) is considered to be a key molecule involved in rapid growth, with the strongest expression in the cartilage layer. However, it remains to be clarified whether deer TGF-β1 has significantly different function from other species such as mouse, and what is the molecular mechanism of regulating cartilage growth.
Methods
Primary chondrocytes was collected from new born mouse rib cartilage. The effect of TGF-β1 on primary chondrocytes viability was elucidated by RNA sequencing (RNA-seq) technology combined with validation methods such as quantitative real-time polymerase chain reaction (qRT-PCR) and immunofluorescence assay (IFA). Differential expression genes were identified using the DEGseq package.
Results
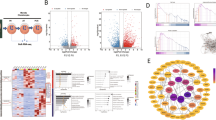
Our results demonstrated that the overexpression of deer TGF-β1 possibly promoted chondrocyte proliferation and extracellular matrix (ECM) synthesis, while simultaneously suppressing chondrocyte differentiation through regulating transcription factors, growth factors, ECM related genes, proliferation and differentiation marker genes, such as Comp, Fgfr3, Atf4, Stat1 etc., and signaling pathways such as the MAPK signaling pathway, inflammatory mediator regulation of TRP channels etc. In addition, by comparing the amino acid sequence and structures between the deer TGF-β1 and mouse TGF-β1, we found that deer TGF-β1 and mouse TGF-β1 proteins are mainly structurally different in arm domains, which is the main functional domain. Phenotypic identification results showed that deer TGF-β1 may has stronger function than mouse TGF-β1.
Conclusion
These results suggested that deer TGF-β1 has the ability to promote chondrogenesis by regulating chondrocyte proliferation, differentiation and ECM synthesis. This study provides insights into the molecular mechanisms underlying the effects of deer TGF-β1 on chondrocyte viability.




Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- TGF-β1:
-
Transforming growth factorβ
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- IFA:
-
Immunofluorescence assay
- ECM:
-
Extracellular matrix
- OA:
-
Osteoarthritis
- RNA-seq:
-
RNA sequencing
- OD:
-
Optical density
- SRA:
-
Sequence read archive
- HISAT:
-
Hierarchical indexing for spliced alignment of transcripts
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- GO:
-
Gene ontology
- OE:
-
Overexpression
- NGF:
-
Nerve growth factor
- IGF-1:
-
Insulin‐like growth factor 1
- BMSCs:
-
Mesenchymal stem cells
- HSP70:
-
Heat shock protein 70
References
Jing X, Du T, Li T, Yang X, Wang G, Liu X et al (2021) The detrimental effect of iron on OA chondrocytes: importance of pro-inflammatory cytokines induced iron influx and oxidative stress. J Cell Mol Med 25(12):5671–5680
Matsushita T, Wilcox WR, Chan YY, Kawanami A, Bukulmez H, Balmes G et al (2009) FGFR3 promotes synchondrosis closure and fusion of ossification centers through the MAPK pathway. Hum Mol Genet 18(2):227–240
Lin R, Deng C, Li X, Liu Y, Zhang M, Qin C et al (2019) Copper-incorporated bioactive glass-ceramics inducing anti-inflammatory phenotype and regeneration of cartilage/bone interface. Theranostics 9(21):6300–6313
Gori I, George R, Purkiss AG, Strohbuecker S, Randall RA, Ogrodowicz R et al (2021) Mutations in SKI in Shprintzen–Goldberg syndrome lead to attenuated TGF-beta responses through SKI stabilization. Elife 10:e63545
Yao B, Zhang M, Leng X, Liu M, Liu Y, Hu Y et al (2018) Antler extracts stimulate chondrocyte proliferation and possess potent anti-oxidative, anti-inflammatory, and immune-modulatory properties. In Vitro Cell Dev Biol Anim 54(6):439–448
Yao B, Zhao Y, Wang Q, Zhang M, Liu M, Liu H et al (2012) De novo characterization of the antler tip of chinese Sika deer transcriptome and analysis of gene expression related to rapid growth. Mol Cell Biochem 364(1–2):93–100
Liu M, Han X, Liu H, Chen D, Li Y, Hu W (2019) The effects of CRISPR-Cas9 knockout of the TGF-beta1 gene on antler cartilage cells in vitro. Cell Mol Biol Lett 24:44
Zhou Z, Yao B, Zhao D (2020) Runx3 regulates chondrocyte phenotype by controlling multiple genes involved in chondrocyte proliferation and differentiation. Mol Biol Rep 47(8):5773–5792
Elbediwy A, Zhang Y, Cobbaut M, Riou P, Tan RS, Roberts SK et al (2019) The rho family GEF FARP2 is activated by aPKCiota to control tight junction formation and polarity. J Cell Sci 132(8):jcs223743
Long HK, Osterwalder M, Welsh IC, Hansen K, Davies JOJ, Liu YE et al (2020) Loss of extreme long-range enhancers in human neural crest drives a craniofacial disorder. Cell Stem Cell 27(5):765-783e714
Dong H, Su A, Lv D, Ma L, Dong J, Guo N et al (2019) Development of whole-porcine monoclonal antibodies with potent neutralization activity against classical swine fever virus from single B cells. ACS Synth Biol 8(5):989–1000
Singhania A, Graham CM, Gabrysova L, Moreira-Teixeira L, Stavropoulos E, Pitt JM et al (2019) Transcriptional profiling unveils type I and II interferon networks in blood and tissues across diseases. Nat Commun 10(1):2887
Robert X, Gouet P (2014) Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res 42:320–324
Hinck AP, Mueller TD, Springer TA (2016) Structural biology and evolution of the TGF-beta family. Cold Spring Harb Perspect Biol 8(12):A202103
Park J, Jeon B, Kang S, Oh M, Kim M, Jang S et al (2015) Study on the changes in enzyme and insulin-like growth Factor-1 concentrations in blood serum and growth characteristics of Velvet Antler during the Antler Growth Period in Sika deer (Cervus nippon). Asian-Australas J Anim Sci 28(9):1303–1308
Chen JC, Hsiang CY, Lin YC, Ho TY (2014) Deer antler extract improves fatigue effect through altering the expression of genes related to muscle strength in skeletal muscle of mice. Evid Based Complement Alternat Med 2014:540580
Ma L, Yang ZQ, Ding JL, Liu S, Guo B, Yue ZP (2019) Function and regulation of transforming growth factor beta1 signalling in antler chondrocyte proliferation and differentiation. Cell Prolif 52(4):e12637
Jurikova M, Danihel L, Polak S, Varga I (2016) Ki67, PCNA, and MCM proteins: markers of proliferation in the diagnosis of breast cancer. Acta Histochem 118(5):544–552
Liao W, Li Z, Zhang H, Li J, Wang K, Yang Y (2015) Proteomic analysis of synovial fluid as an analytical tool to detect candidate biomarkers for knee osteoarthritis. Int J Clin Exp Pathol 8(9):9975–9989
Arredouani M, Matthijs P, Van Hoeyveld E, Kasran A, Baumann H, Ceuppens JL et al (2003) Haptoglobin directly affects T cells and suppresses T helper cell type 2 cytokine release. Immunology 108(2):144–151
Yang H, Zhang M, Liu Q, Zhang H, Zhang J, Lu L et al (2019) Inhibition of IHH reverses temporomandibular joint osteoarthritis via a PTH1R signaling dependent mechanism. Int J Mol Sci 20(15):3797
Zhang W, Xue D, Yin H, Wang S, Li C, Chen E et al (2016) Overexpression of HSPA1A enhances the osteogenic differentiation of bone marrow mesenchymal stem cells via activation of the Wnt/beta-catenin signaling pathway. Sci Rep 6:27622
Li C, Sunderic K, Nicoll SB, Wang S (2018) Downregulation of heat shock protein 70 impairs osteogenic and chondrogenic differentiation in human mesenchymal stem cells. Sci Rep 8(1):553
Lv S, Xu J, Chen L, Wu H, Feng W, Zheng Y et al (2020) MicroRNA-27b targets CBFB to inhibit differentiation of human bone marrow mesenchymal stem cells into hypertrophic chondrocytes. Stem Cell Res Ther 11(1):392
Foster JW, Dominguez-Steglich MA, Guioli S, Kwok C, Weller PA, Stevanovic M et al (1994) Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 372(6506):525–530
Haseeb A, Kc R, Angelozzi M, de Charleroy C, Rux D, Tower RJ et al (2021) SOX9 keeps growth plates and articular cartilage healthy by inhibiting chondrocyte dedifferentiation/osteoblastic redifferentiation. Proc Natl Acad Sci USA 118(8):e2019152118
Wang W, Lian N, Li L, Moss HE, Wang W, Perrien DS et al (2009) Atf4 regulates chondrocyte proliferation and differentiation during endochondral ossification by activating Ihh transcription. Development 136(24):4143–4153
Keppie SJ, Mansfield JC, Tang X, Philp CJ, Graham HK, Onnerfjord P et al (2021) Matrix-bound growth factors are released upon cartilage compression by an aggrecan-dependent sodium flux that is lost in osteoarthritis. Function (Oxf) 2(5):zqab037
Zelzer E, Mamluk R, Ferrara N, Johnson RS, Schipani E, Olsen BR (2004) VEGFA is necessary for chondrocyte survival during bone development. Development 131(9):2161–2171
Uchimura T, Hollander JM, Nakamura DS, Liu Z, Rosen CJ, Georgakoudi I et al (2017) An essential role for IGF2 in cartilage development and glucose metabolism during postnatal long bone growth. Development 144(19):3533–3546
He J, Zhang J, Wang D (2017) Down-regulation of microRNA-216b inhibits IL-1beta-induced chondrocyte injury by up-regulation of Smad3. Biosci Rep 37(2):588
Acknowledgements
This research was supported by Jilin Province Young and Middle-aged Science and Technology Innovation and Entrepreneurship Outstanding Talent (team) Project (No. 20210509001RQ), National Natural Science Foundation of China (No. 81973887), and Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (No. ZYYCXTD-D-202001).
Funding
This research was supported by Jilin Province Young and Middle-aged Science and Technology Innovation and Entrepreneurship Outstanding Talent (team) Project (No. 20210509001RQ), National Natural Science Foundation of China (No. 81973887), and Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (No. ZYYCXTD-D-202001).
Author information
Authors and Affiliations
Contributions
ZZ, HD and XW: Manuscript writing. ZZ, JZ, JZ, JY: Experiment conduction and data analysis. HD, BY, XL: Manuscript subject design.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Ethical approval
All procedures were performed in accordance with the guidelines of the Institutional Animal Ethics Committee of Changchun University of Chinese Medicine (No. ccucm-2017-0015).
Consent to participate and for publication
The authors approved the manuscript to be submitted for consideration for publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Z., Zhong, J., Zhang, J. et al. Comparative transcriptome analysis provides insight into the molecular targets and signaling pathways of deer TGF-1 regulating chondrocytes proliferation and differentiation. Mol Biol Rep 50, 3155–3166 (2023). https://doi.org/10.1007/s11033-023-08265-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08265-z