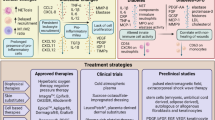
Abstract
Diabetes is a complex of genetic, metabolic, and autoimmune disorders that are characterized by hyperglycemia. Elevated apoptotic cell count following defective clearance of dead cells that can cause chronic inflammation is a hallmark of the diabetic wound. Effective dead cell clearance is a prerequisite for rapid inflammation resolution and successful recovery. Efferocytosis is a multistep process in which phagocytes engulf the dead cells. Cell body elimination is of great significance in disease and homeostasis. Recent research has clarified that diabetic wounds have an enhanced load of the apoptotic cell, which is partly attributed to the dysfunction of macrophages in apoptotic clearance at the site of the diabetic wounds. In the current work, we highlight the pathways implicated in efferocytosis, from the diagnosis of apoptotic cells to the phagocytic swallowing and the homeostatic resolution, and explain the possible pathophysiological episodes occurring when the proceeding is abrogated. Also, we describe the last development in the management of inflammation in diabetes wound and future directions of surveillance.





Similar content being viewed by others
References
Rajkumar V, Levine SN (2022) Latent Autoimmune Diabetes, in StatPearls. StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.: Treasure Island (FL)
Bardsley JK, Want LL (2004) Overview of diabetes. Crit Care Nurs Q 27(2):106–112
De Freitas GR et al (2021) Dry eyes in patients with diabetes mellitus. Prim Care Diabetes 15(1):184–186
von Scholten BJ et al (2021) Current and future therapies for type 1 diabetes. Diabetologia 64(5):1037–1048
Atkinson MA, Eisenbarth GS (2001) Type 1 diabetes: new perspectives on disease pathogenesis and treatment. The Lancet 358(9277):221–229
Zhou B et al (2016) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4· 4 million participants. The Lancet 387(10027):1513–1530
Chatterjee S, Khunti K, Davies MJ (2017) Type 2 diabetes. The Lancet 389(10085):2239–2251
Marx N et al (2021) Guideline recommendations and the positioning of newer drugs in type 2 diabetes care. Lancet Diabetes Endocrinol 9(1):46–52
Coustan DR (2013) Gestational diabetes mellitus. Clin Chem 59(9):1310–1321
McIntyre HD et al (2019) Gestational diabetes mellitus. Nat reviews Disease primers 5(1):1–19
Kerr JF (2002) History of the events leading to the formulation of the apoptosis concept. Toxicology 181:471–474
King K, Cidlowski J (1998) Cell cycle regulation and apoptosis. Annu Rev Physiol 60(1):601–617
Cotter T, deCathelineau AM, Henson PM (2003) The final step in programmed cell death: phagocytes carry apoptotic cells to the grave. Essays Biochem 39:105–117
Peter C et al (2010) Dangerous attraction: phagocyte recruitment and danger signals of apoptotic and necrotic cells. Apoptosis 15(9):1007–1028
Arandjelovic S, Ravichandran KS (2015) Phagocytosis of apoptotic cells in homeostasis. Nat Immunol 16(9):907–917
Poon IK et al (2014) Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol 14(3):166–180
D’Arcy MS (2019) Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int 43(6):582–592
Lauber K et al (2003) Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell 113(6):717–730
Elliott MR et al (2009) Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461(7261):282–286
Truman LA et al (2008) CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood The Journal of the American Society of Hematology 112(13):5026–5036
Abdolmaleki F et al (2018) The role of efferocytosis in autoimmune diseases. Front Immunol 9:1645
Elliott MR, Koster KM, Murphy PS (2017) Efferocytosis signaling in the regulation of macrophage inflammatory responses. J Immunol 198(4):1387–1394
Segawa K, Nagata S (2015) An apoptotic ‘eat me’signal: phosphatidylserine exposure. Trends Cell Biol 25(11):639–650
Kim SJ et al (2002) I-PLA2 activation during apoptosis promotes the exposure of membrane lysophosphatidylcholine leading to binding by natural immunoglobulin M antibodies and complement activation. J Exp Med 196(5):655–665
Gardai SJ et al (2005) Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123(2):321–334
Hochreiter-Hufford A, Ravichandran KS (2013) Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb Perspect Biol 5(1):a008748
Wu Y, Tibrewal N, Birge RB (2006) Phosphatidylserine recognition by phagocytes: a view to a kill. Trends Cell Biol 16(4):189–197
Richards DM, Endres RG (2014) The mechanism of phagocytosis: two stages of engulfment. Biophys J 107(7):1542–1553
Rosales C, Uribe-Querol E (2017) Phagocytosis: a fundamental process in immunity. BioMed research international, 2017
Ma Z et al (2002) Regulation of Rac1 activation by the low density lipoprotein receptor–related protein. J Cell Biol 159(6):1061–1070
Wang L et al (2020) Potential Mechanisms and Effects of Efferocytosis in Atherosclerosis. Front Endocrinol (Lausanne) 11:585285
Rink J et al (2005) Rab conversion as a mechanism of progression from early to late endosomes. Cell 122(5):735–749
Boada-Romero E et al (2020) The clearance of dead cells by efferocytosis. Nat Rev Mol Cell Biol 21(7):398–414
Werfel TA, Cook RS (2018) Efferocytosis in the tumor microenvironment. Semin Immunopathol 40(6):545–554
Mathis D, Vence L, Benoist C (2001) β-Cell death during progression to diabetes. Nature, 414(6865): p. 792–798
Vives-Pi M, Rodríguez-Fernández S, Pujol-Autonell I (2015) How apoptotic β-cells direct immune response to tolerance or to autoimmune diabetes: a review. Apoptosis 20(3):263–272
Morioka S, Maueröder C, Ravichandran KS (2019) Living on the Edge: Efferocytosis at the Interface of Homeostasis and Pathology. Immunity 50(5):1149–1162
Doran AC, Yurdagul A Jr, Tabas I (2020) Efferocytosis in health and disease 20(4):254–267
Thorp E et al (2011) Shedding of the Mer tyrosine kinase receptor is mediated by ADAM17 protein through a pathway involving reactive oxygen species, protein kinase Cδ, and p38 mitogen-activated protein kinase (MAPK). J Biol Chem 286(38):33335–33344
O’Brien BA et al (2002) Clearance of apoptotic β-cells is reduced in neonatal autoimmune diabetes-prone rats. Cell Death & Differentiation 9(4):457–464
Marée AF et al (2005) Quantifying macrophage defects in type 1 diabetes. J Theor Biol 233(4):533–551
Goren I et al (2007) Systemic anti-TNFα treatment restores diabetes-impaired skin repair in ob/ob mice by inactivation of macrophages. J Invest Dermatology 127(9):2259–2267
Tabas I (2005) Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arteriosclerosis, thrombosis, and vascular biology. 25:2255–226411
Thorp E, Tabas I (2009) Mechanisms and consequences of efferocytosis in advanced atherosclerosis. J Leukoc Biol 86(5):1089–1095
Naghavi M et al (2003) From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation 108(14):1664–1672
Fredman G et al (2015) Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci Transl Med 7(275):275ra20
Martinez RM et al (2018) Lipoxin A4 inhibits UV radiation-induced skin inflammation and oxidative stress in mice.J Dermatol Sci,
Viola JR et al (2016) Resolving Lipid Mediators Maresin 1 and Resolvin D2 Prevent Atheroprogression in Mice. Circ Res 119(9):1030–1038
An Y et al (2019) Activation of ROS/MAPKs/NF-κB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 33:12515–1252711
Das A et al (2016) Correction of MFG-E8 resolves inflammation and promotes cutaneous wound healing in diabetes. J Immunol 196(12):5089–5100
Saevarsdottir S, Vikingsdottir T, Valdimarsson H (2004) The potential role of mannan-binding lectin in the clearance of self-components including immune complexes. Scand J Immunol 60(1–2):23–29
Nauta AJ et al (2003) Mannose-binding lectin engagement with late apoptotic and necrotic cells. Eur J Immunol 33(10):2853–2863
Saevarsdottir S et al (2005) Mannan binding lectin as an adjunct to risk assessment for myocardial infarction in individuals with enhanced risk. J Exp Med 201(1):117–125
Yamauchi T et al (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423(6941):762–769
Takemura Y et al (2007) Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest 117(2):375–386
Cash JG et al (2012) Apolipoprotein E4 impairs macrophage efferocytosis and potentiates apoptosis by accelerating endoplasmic reticulum stress. J Biol Chem 287(33):27876–27884
An Y et al (2019) Activation of ROS/MAPKs/NF-κB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis. Faseb j 33(11):12515–12527
Kwon CH et al (2020) Clinically confirmed DEL-1 as a myokine attenuates lipid-induced inflammation and insulin resistance in 3T3-L1 adipocytes via AMPK/HO-1- pathway. Adipocyte 9(1):576–586
Wu Y, Tibrewal N, Birge RB (2006) Phosphatidylserine recognition by phagocytes: a view to a kill. Trends Cell Biol 16(4):189–197
Ge Y, Huang M, Yao YM (2022) Efferocytosis and Its Role in Inflammatory Disorders. Front Cell Dev Biol 10:839248
Bitzan M et al (2008) Rituximab treatment of collapsing C1q glomerulopathy: Clinical and histopathological evolution. Pediatr Nephrol 23(8):1355–1361
Chao MP et al (2010) Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 142(5):699–713
Albert ML et al (1998) Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med 188(7):1359–1368
Greenberg ME et al (2006) Oxidized phosphatidylserine–CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med 203(12):2613–2625
Hijazi H, Waggiallah H, Alagib A (2013) Oxidative low density lipoprotien prohibited plasmodium falciparum clearance in type 2 diabetes mellitus via cluster differentiation 36. North Am J Med Sci 5(12):703–706
Sóñora C et al (2014) Anti-tissue transglutaminase antibody inhibits apoptotic cell clearance by macrophages in pregnant NOD mice. J Reprod Immunol 103(1):59–66
Eizirik DL, Colli ML, Ortis F (2009) The role of inflammation in insulitis and β-cell loss in type 1 diabetes. Nat Reviews Endocrinol 5(4):219–226
Villalba A et al (2020) Preclinical evaluation of antigen-specific nanotherapy based on phosphatidylserine-liposomes for type 1 diabetes. Artif Cells Nanomed Biotechnol 48(1):77–83
Pujol-Autonell I et al (2015) Use of autoantigen-loaded phosphatidylserine-liposomes to arrest autoimmunity in type 1 diabetes.PLoS ONE, 10(6)
Rodriguez-Fernandez S et al (2018) Phosphatidylserine-liposomes promote tolerogenic features on dendritic cells in human type 1 diabetes by apoptotic mimicry. Frontiers in Immunology, 9(FEB)
Lugovaya A et al (2019) Spontaneous and activation-induced apoptosis of peripheral blood mononuclear cells in the pathogenesis of type 1 diabetes mellitus. Med Immunol (Russia) 22(1):123–134
Lugovaya AV et al (2020) Spontaneous and activation-induced apoptosis of peripheral blood mononuclear cells in the pathogenesis of type 1 diabetes mellitus. Med Immunol (Russia) 22(1):123–134
Kakarla R et al (2020) Apoptotic cell-derived exosomes: messages from dying cells. Exp Mol Med 52(1):1–6
Zheng C et al (2021) Apoptotic vesicles restore liver macrophage homeostasis to counteract type 2 diabetes. J Extracell Vesicles 10(7):e12109
Hie M, Yamazaki M, Tsukamoto I (2009) Curcumin suppresses increased bone resorption by inhibiting osteoclastogenesis in rats with streptozotocin-induced diabetes. Eur J Pharmacol 621(1–3):1–9
Saito M, Marumo K (2010) Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int 21(2):195–214
Davies LC et al (2013) Tissue-resident macrophages. Nat Immunol 14(10):986–995
Soehnlein O, Lindbom L (2010) Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol 10(6):427–439
Bain S et al (1997) Tetracycline prevents cancellous bone loss and maintains near-normal rates of bone formation in streptozotocin diabetic rats. Bone 21(2):147–153
An Y et al (2019) Activation of ROS/MAPKs/NF-κB/NLRP3 and inhibition of efferocytosis in osteoclast‐mediated diabetic osteoporosis. FASEB J 33(11):12515–12527
Green D, Oguin T, Martinez J (2016) The clearance of dying cells: table for two. Cell Death & Differentiation 23(6):915–926
Kantharidis P et al (2011) Diabetes complications: the microRNA perspective. Diabetes 60(7):1832–1837
Regazzi R (2018) MicroRNAs as therapeutic targets for the treatment of diabetes mellitus and its complications. Expert Opin Ther Targets 22(2):153–160
Roy S (2016) miRNA in Macrophage Development and Function. Antioxid Redox Signal 25(15):795–804
Singh RP et al (2013) The role of miRNA in inflammation and autoimmunity. Autoimmun Rev 12(12):1160–1165
Sen CK, Ghatak S (2015) miRNA control of tissue repair and regeneration. Am J Pathol 185(10):2629–2640
Kim SY, Nair MG (2019) Macrophages in wound healing: activation and plasticity. Immunol Cell Biol 97(3):258–267
Das A et al (2016) Correction of MFG-E8 Resolves Inflammation and Promotes Cutaneous Wound Healing. Diabetes 196(12):5089–5100
Sinha M et al (2018) Direct conversion of injury-site myeloid cells to fibroblast-like cells of granulation tissue.Nature Communications, 9(1)
Dastah S et al (2020) Aerobic exercise leads to upregulation of Mir-126 and angiogenic signaling in the heart tissue of diabetic rats.Gene Reports, 21
Banerjee J et al (2020) Senescence-associated miR-34a and miR-126 in middle-aged Indians with type 2 diabetes. Clin Experimental Med 20(1):149–158
Liu Y et al (2014) The role of circulating microRNA-126 (miR-126): a novel biomarker for screening prediabetes and newly diagnosed type 2 diabetes mellitus. Int J Mol Sci 15(6):10567–10577
Zampetaki A et al (2010) Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res 107(6):810–817
Wang C et al (2017) miR-126-5p Restoration Promotes Cell Apoptosis in Cervical Cancer by Targeting Bcl2l2. Oncol Res 25(4):463–470
Suresh Babu S et al (2016) MicroRNA-126 overexpression rescues diabetes-induced impairment in efferocytosis of apoptotic cardiomyocytes.Scientific Reports, 6
Sather S et al (2007) A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood 109(3):1026–1033
Krause S et al (2015) Mer tyrosine kinase promotes the survival of t(1;19)-positive acute lymphoblastic leukemia (ALL) in the central nervous system (CNS). Blood 125(5):820–830
Toda S, Segawa K, Nagata S (2014) MerTK-mediated engulfment of pyrenocytes by central macrophages in erythroblastic islands. Blood 123(25):3963–3971
Cai B et al (2020) Macrophage MerTK Promotes Liver Fibrosis in Nonalcoholic Steatohepatitis. Cell Metabol 31(2):406–421e7
Choi JY et al (2013) Upregulation of Mer receptor tyrosine kinase signaling attenuated lipopolysaccharide-induced lung inflammation. J Pharmacol Exp Ther 344(2):447–458
Uchiyama A et al (2017) Mesenchymal stem cells-derived MFG-E8 accelerates diabetic cutaneous wound healing. J Dermatol Sci 86(3):187–197
Khanna S et al (2010) Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS ONE 5(3):e9539
Barman PK, Koh TJ (2020) Macrophage Dysregulation and Impaired Skin Wound Healing in Diabetes. Front cell Dev biology 8:528–528
Li K et al (2021) MRP8/14 mediates macrophage efferocytosis through RAGE and Gas6/MFG-E8, and induces polarization via TLR4-dependent pathway. 236:1375–13902
Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414(6865):813–820
Kim SY et al (2022) Efferocytosis and enhanced FPR2 expression following apoptotic cell instillation attenuate radiation-induced lung inflammation and fibrosis. Biochem Biophys Res Commun 601:38–44
Krzyszczyk P et al (2018) The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol 9:419
Yan J et al (2018) Diabetes impairs wound healing by Dnmt1-dependent dysregulation of hematopoietic stem cells differentiation towards macrophages. Nat Commun 9(1):1–13
Mirza RE et al (2013) Blocking interleukin-1β induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes 62(7):2579–2587
Wang Q et al (2017) Blocking AGE-RAGE signaling improved functional disorders of macrophages in diabetic wound. Journal of diabetes research, 2017
Gallagher KA et al (2015) Epigenetic changes in bone marrow progenitor cells influence the inflammatory phenotype and alter wound healing in type 2 diabetes. Diabetes 64(4):1420–1430
Yang F et al (2010) Essential role for Smad3 in angiotensin II-induced tubular epithelial–mesenchymal transition. J Pathol 221(4):390–401
Barman PK, Koh TJ (2020) Macrophage dysregulation and impaired skin wound healing in diabetes. Front Cell Dev Biology 8:528
Chen H et al (2015) Macrophage peroxisome proliferator-activated receptor γ deficiency delays skin wound healing through impairing apoptotic cell clearance in mice. Cell Death Dis 6(1):e1597
Mirza RE et al (2015) Macrophage PPARγ and impaired wound healing in type 2 diabetes. J Pathol 236(4):433–444
Wang Q et al (2017) Blocking AGE-RAGE Signaling Improved Functional Disorders of Macrophages in Diabetic Wound. 2017:1428537
Chatzigeorgiou A et al (2010) The pattern of inflammatory/anti-inflammatory cytokines and chemokines in type 1 diabetic patients over time. Ann Med 42(6):426–438
Uchiyama A et al (2015) Protective effect of MFG-E8 after cutaneous ischemia–reperfusion injury. J Invest dermatology 135(4):1157–1165
Jun JI, Kim KH, Lau LF (2015) The matricellular protein CCN1 mediates neutrophil efferocytosis in cutaneous wound healing.Nature Communications, 6
Lima MH et al (2012) Topical insulin accelerates wound healing in diabetes by enhancing the AKT and ERK pathways: a double-blind placebo-controlled clinical trial. PLoS ONE 7(5):e36974
Huang JJ et al (2020) Annexin A1-derived peptide Ac2‐26 facilitates wound healing in diabetic mice. Wound Repair and Regeneration 28(6):772–779
Huang JJ et al (2020) Annexin A1-derived peptide Ac2-26 facilitates wound healing in diabetic mice. Wound Repair and Regeneration 28(6):772–779
Chen X, Liu Y, Zhang X (2012) Topical insulin application improves healing by regulating the wound inflammatory response. Wound Repair and Regeneration 20(3):425–434
Yang P et al (2021) Topical insulin application accelerates diabetic wound healing by promoting anti-inflammatory macrophage polarization.Journal of Cell Science, 133(19)
Gullo D et al (2021) Insulin autoimmune syndrome misdiagnosed as an insulinoma in a woman presenting with a pancreatic cystic lesion and taking alpha lipoic acid: a lesson to be learned. Hormones 20(3):593–595
Erdem N, Montero E, Roep BO (2021) Breaking and restoring immune tolerance to pancreatic beta-cells in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes 28(4):397–403
Wang L, Wang FS, Gershwin ME (2015) Human autoimmune diseases: a comprehensive update. J Intern Med 278(4):369–395
Klein L, Robey EA, Hsieh C-S (2019) Central CD4 + T cell tolerance: deletion versus regulatory T cell differentiation. Nat Rev Immunol 19(1):7–18
Malhotra D et al (2016) Tolerance is established in polyclonal CD4 + T cells by distinct mechanisms, according to self-peptide expression patterns. Nat Immunol 17(2):187–195
Baccala R et al (2007) TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med 13(5):543–551
Mitchell S et al (2002) Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J Am Soc Nephrol 13(10):2497–2507
Libreros S et al (2020) A New E-Series Resolvin: RvE4 Stereochemistry and Function in Efferocytosis of Inflammation-Resolution. Front Immunol 11:631319
Milligan G, Stoddart LA, Brown AJ (2006) G protein-coupled receptors for free fatty acids. Cell Signal 18(9):1360–1365
Peterson EA, Sun J (2022) Leukocyte-Mediated Cardiac Repair after Myocardial Infarction in Non-Regenerative vs.Regenerative Systems.9(2)
Vandendriessche S et al (2021) Complement Receptors and Their Role in Leukocyte Recruitment and Phagocytosis. Front Cell Dev Biol 9:624025
Abdolmaleki F et al (2020) Resolvins: emerging players in autoimmune and inflammatory diseases. Clin Rev Allergy Immunol 58(1):82–91
Halban PA (1991) Structural domains and molecular lifestyles of insulin and its precursors in the pancreatic beta cell. Diabetologia 34(11):767–778
Martinon F et al (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440(7081):237–241
Prencipe G et al (2014) Inflammasome activation by cystine crystals: implications for the pathogenesis of cystinosis. J Am Soc Nephrol 25(6):1163–1169
Engelen-Lee JY et al (2018) Histopathology of Listeria meningitis. J Neuropathol Exp Neurol 77(10):950–957
Suresh Babu S et al (2016) MicroRNA-126 overexpression rescues diabetes-induced impairment in efferocytosis of apoptotic cardiomyocytes. Sci Rep 6(1):36207
Mahmoudi A et al (2022) MicroRNAs and Efferocytosis: Implications for Diagnosis and Therapy. Mini Rev Med Chem
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There was no conflict of interest in the current study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mahmoudi, A., firouzjaei, A.A., darijani, F. et al. Effect of diabetes on efferocytosis process. Mol Biol Rep 49, 10849–10863 (2022). https://doi.org/10.1007/s11033-022-07725-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07725-2