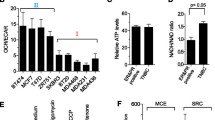
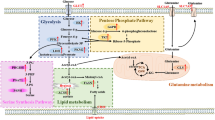
Abstract
Among breast cancer subtypes, the triple negative breast cancer (TNBC) has the worst prognosis. In absence of any permitted targeted therapy, standard chemotherapy is the mainstay for TNBC treatment. Hence, there is a crucial need to identify potential druggable targets in TNBCs for its effective treatment. In recent times, metabolic reprogramming has emerged as cancer cells hallmark, wherein cancer cells display discrete metabolic phenotypes to fuel cell progression and metastasis. Altered glycolysis is one such phenotype, in which even in oxygen abundance majority of cancer cells harvest considerable amount of energy through elevated glycolytic-flux. In the present review, we attempt to summarize the role of key glycolytic enzymes i.e. HK, Hexokinase; PFK, Phosphofructokinase; PKM2, Pyruvate kinase isozyme type 2; and LDH, Lactate dehydrogenase in TNBCs, and possible therapeutic options presently available.

Similar content being viewed by others
References
Lebert JM, Lester R, Powell E, Seal M, McCarthy J (2018) Advances in the systemic treatment of triple-negative breast cancer. Curr Oncol 25(Suppl 1):S142–S150
Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L (2016) Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol 13(11):674–690
Gluz O, Liedtke C, Gottschalk N, Pusztai L, Nitz U, Harbeck N (2009) Triple-negative breast cancer–current status and future directions. Ann Oncol 20(12):1913–1927
Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA et al (2008) Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 26(8):1275–1281
Collignon J, Lousberg L, Schroeder H, Jerusalem G (2016) Triple-negative breast cancer: treatment challenges and solutions. Breast Cancer (Dove Med Press) 8:93–107
Lunt SY, Vander Heiden MG (2011) Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 27:441–464
Kroemer G, Pouyssegur J (2008) Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell 13(6):472–482
Warburg O, Wind F, Negelein E (1927) The metabolism of tumors in the body. J Gen Physiol 8(6):519–530
Samec M, Liskova A, Koklesova L, Samuel SM, Zhai K, Buhrmann C et al (2020) Flavonoids against the Warburg phenotype-concepts of predictive, preventive and personalised medicine to cut the Gordian knot of cancer cell metabolism. EPMA J 11(3):377–398
Annibaldi A, Widmann C (2010) Glucose metabolism in cancer cells. Curr Opin Clin NutrMetab Care 13(4):466–470
Altenberg B, Greulich KO (2004) Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics 84(6):1014–1020
Badowska-Kozakiewicz AM, Budzik MP, Przybylski J (2015) Hypoxia in breast cancer. Pol J Pathol 66(4):337–346
Ponente M, Campanini L, Cuttano R, Piunti A, Delledonne GA, Coltella N et al (2017) PML promotes metastasis of triple-negative breast cancer through transcriptional regulation of HIF1A target genes. JCI Insight. 2(4):e87380
Robey IF, Lien AD, Welsh SJ, Baggett BK, Gillies RJ (2005) Hypoxia-inducible factor-1alpha and the glycolytic phenotype in tumors. Neoplasia 7(4):324–330
Lu S, Gu X, Hoestje S, Epner DE (2002) Identification of an additional hypoxia responsive element in the glyceraldehyde-3-phosphate dehydrogenase gene promoter. BiochimBiophys Acta 1574(2):152–156
Kanaan YM, Sampey BP, Beyene D, Esnakula AK, Naab TJ, Ricks-Santi LJ et al (2014) Metabolic profile of triple-negative breast cancer in African-American women reveals potential biomarkers of aggressive disease. Cancer Genomics Proteomics 11(6):279–294
Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB, Hay N (2001) Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev 15(11):1406–1418
Majewski N, Nogueira V, Bhaskar P, Coy PE, Skeen JE, Gottlob K et al (2004) Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell 16(5):819–830
Mathupala SP, Rempel A, Pedersen PL (2001) Glucose catabolism in cancer cells: identification and characterization of a marked activation response of the type II hexokinase gene to hypoxic conditions. J Biol Chem 276(46):43407–43412
Wu Z, Wu J, Zhao Q, Fu S, Jin J (2020) Emerging roles of aerobic glycolysis in breast cancer. Clin Transl Oncol 22(5):631–646
Hennipman A, Smits J, van Oirschot B, van Houwelingen JC, Rijksen G, Neyt JP et al (1987) Glycolytic enzymes in breast cancer, benign breast disease and normal breast tissue. Tumour Biol 8(5):251–263
Hennipman A, van Oirschot BA, Smits J, Rijksen G, Staal GE (1988) Heterogeneity of glycolytic enzyme activity and isozyme composition of pyruvate kinase in breast cancer. Tumour Biol 9(4):178–189
Brown RS, Goodman TM, Zasadny KR, Greenson JK, Wahl RL (2002) Expression of hexokinase II and Glut-1 in untreated human breast cancer. Nucl Med Biol 29(4):443–453
Guha M, Srinivasan S, Raman P, Jiang Y, Kaufman BA, Taylor D et al (2018) Aggressive triple negative breast cancers have unique molecular signature on the basis of mitochondrial genetic and functional defects. Biochim Biophys Acta Mol Basis Dis. 64(4 Pt A):1060–1071
Robey RB, Hay N (2006) Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene 25(34):4683–4696
Coelho RG, Calaça IC, Celestrini DM, Correia-Carneiro AH, Costa MM et al (2015) Hexokinase and phosphofructokinase activity and intracellular distribution correlate with aggressiveness and invasiveness of human breast carcinoma. Oncotarget 6(30):29375–29387
Palmieri D, Fitzgerald D, Shreeve SM, Hua E, Bronder JL, Weil RJ et al (2009) Analyses of resected human brain metastases of breast cancer reveal the association between up-regulation of hexokinase 2 and poor prognosis. Mol Cancer Res 7(9):1438–1445
Sato-Tadano A, Suzuki T, Amari M, Takagi K, Miki Y, Tamaki K et al (2013) Hexokinase II in breast carcinoma: a potent prognostic factor associated with hypoxia-inducible factor-1α and Ki-67. Cancer Sci 104(10):1380–1388
Kaplan O, Jaroszewski JW, Faustino PJ, Zugmaier G, Ennis BW, Lippman M et al (1990) Toxicity and effects of epidermal growth factor on glucose metabolism of MDA-468 human breast cancer cells. J Biol Chem 265(23):13641–13649
Carey L, Winer E, Viale G, Cameron D, Gianni L (2010) Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol 7(12):683–692
Lim SO, Li CW, Xia W, Lee HH, Chang SS, Shen J et al (2016) EGFR signaling enhances aerobic glycolysis in triple-negative breast cancer cells to promote tumor growth and immune escape. Cancer Res 76(5):1284–1296
Zhang D, Wang H, Yu W, Qiao F, Su X, Xu H et al (2019) Downregulation of hexokinase 2 improves radiosensitivity of breast cancer. Trans Cancer Res 8:290–297
Liu X, Miao W, Huang M, Li L, Dai X, Wang Y (2019) Elevated hexokinase II expression confers acquired resistance to 4-hydroxytamoxifen in breast cancer cells. Mol Cell Proteomics 18(11):2273–2284
Al Hasawi N, Alkandari MF, Luqmani YA (2014) Phosphofructokinase: a mediator of glycolytic flux in cancer progression. Crit Rev Oncol Hematol 92(3):312–321
Jenkins CM, Yang J, Sims HF, Gross RW (2011) Reversible high affinity inhibition of phosphofructokinase-1 by acyl-CoA: a mechanism integrating glycolytic flux with lipid metabolism. J Biol Chem 286(14):11937–11950
Zancan P, Rosas AO, Marcondes MC, Marinho-Carvalho MM, Sola-Penna M (2007) Clotrimazole inhibits and modulates heterologous association of the key glycolytic enzyme 6-phosphofructo-1-kinase. BiochemPharmacol 73(10):1520–1527
Moreno-Sánchez R, Rodríguez-Enríquez S, Marín-Hernández A, Saavedra E (2007) Energy metabolism in tumor cells. FEBS J 274(6):1393–1418
Enzo E, Santinon G, Pocaterra A, Aragona M, Bresolin S, Forcato M et al (2015) Aerobic glycolysis tunes YAP/TAZ transcriptional activity. EMBO J 34(10):1349–1370
Zancan P, Sola-Penna M, Furtado CM, Da Silva D (2010) Differential expression of phosphofructokinase-1 isoforms correlates with the glycolytic efficiency of breast cancer cells. Mol Genet Metab 100(4):372–378
Wang G, Xu Z, Wang C, Yao F, Li J, Chen C et al (2013) Differential phosphofructokinase-1 isoenzyme patterns associated with glycolytic efficiency in human breast cancer and paracancer tissues. Oncol Lett 6(6):1701–1706
Prasad CP, Södergren K, Andersson T (2017) Reduced production and uptake of lactate are essential for the ability of WNT5A signaling to inhibit breast cancer cell migration and invasion. Oncotarget 8(42):71471–71488
Moon JS, Kim HE, Koh E, Park SH, Jin WJ, Park BW et al (2011) Krüppel-like factor 4 (KLF4) activates the transcription of the gene for the platelet isoform of phosphofructokinase (PFKP) in breast cancer. J Biol Chem 286(27):23808–23816
Peng M, Yang D, Hou Y, Liu S, Zhao M, Qin Y et al (2019) Intracellular citrate accumulation by oxidized ATM-mediated metabolism reprogramming via PFKP and CS enhances hypoxic breast cancer cell invasion and metastasis. Cell Death Dis 10(3):228
Yeerken D, Hong R, Wang Y, Gong Y, Liu R, Yang D et al (2020) PFKP is transcriptionally repressed by BRCA1/ZBRK1 and predicts prognosis in breast cancer. PLoS One. 15(5):e00233750
Going CC, Tailor D, Kumar V, Birk AM, Pandrala M, Rice MA et al (2018) Quantitative proteomic profiling reveals key pathways in the anticancer action of methoxychalcone derivatives in triple negative breast cancer. J Proteome Res 17(10):3574–3585
Barupal DK, Gao B, Budczies J, Phinney BS, Perroud B, Denkert C et al (2019) Prioritization of metabolic genes as novel therapeutic targets in estrogen-receptor negative breast tumors using multi-omics data and text mining. Oncotarget 10(39):3894–3909
Mazurek S (2011) Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol 43(7):969–980
Wong N, De Melo J, Tang D (2013) PKM2, a central point of regulation in cancer metabolism. Int J Cell Biol. 23:242513
Mazurek S, Boschek CB, Hugo F, Eigenbrodt E (2005) Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol 15(4):300–308
Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R et al (2008) The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452(7184):230–233
Su Q, Luo S, Tan Q, Deng J, Zhou S, Peng M et al (2019) The role of pyruvate kinase M2 in anticancer therapeutic treatments. Oncol Lett 18(6):5663–5672
Benesch C, Schneider C, Voelker HU, Kapp M, Caffier H, Krockenberger M et al (2010) The clinicopathological and prognostic relevance of pyruvate kinase M2 and pAkt expression in breast cancer. Anticancer Res 30(5):1689–1694
Wang Y, Liu J, Jin X, Zhang D, Li D, Hao F et al (2017) O-GlcNAcylation destabilizes the active tetrameric PKM2 to promote the Warburg effect. Proc Natl Acad Sci U S A 114(52):13732–13737
Lin Y, Lv F, Liu F, Guo X, Fan Y, Gu F et al (2015) High Expression of pyruvate kinase M2 is associated with chemosensitivity to epirubicin and 5-fluorouracil in breast cancer. J Cancer 6(11):1130–1139
Zhang L, Bailleul J, Yazal T, Dong K, Sung D, Dao A et al (2019) PK-M2-mediated metabolic changes in breast cancer cells induced by ionizing radiation. Breast Cancer Res Treat 178(1):75–86
Dong G, Mao Q, Xia W, Dong K, Sung D, Dao A et al (2016) PKM2 and cancer: the function of PKM2 beyond glycolysis. Oncol Lett 11(3):1980–1986
Shen J, Liu H, Mu C, Wolfram J, Zhang W, Kim HC et al (2017) Multi-step encapsulation of chemotherapy and gene silencing agents in functionalized mesoporous silica nanoparticles. Nanoscale 9(16):5329–5341
Zhou Z, Li M, Zhang L, Zhao H, Şahin Ö, Chen J et al (2018) Oncogenic kinase-induced PKM2 tyrosine 105 phosphorylation converts nononcogenic PKM2 to a tumor promoter and induces cancer stem-like cells. Cancer Res 78(9):2248–2261
Ma C, Zu X, Liu K, Bode AM, Dong Z, Liu Z et al (2019) Knockdown of pyruvate kinase M inhibits cell growth and migration by reducing NF-kB activity in triple-negative breast cancer cells. Mol Cells 42(9):628–636
Valvona CJ, Fillmore HL, Nunn PB, Pilkington GJ (2016) The regulation and function of lactate dehydrogenase a: therapeutic potential in brain tumor. Brain Pathol 26(1):3–17
Markert CL, Shaklee JB, Whitt GS (1975) Evolution of a gene. Multiple genes for LDH isozymes provide a model of the evolution of gene structure, function and regulation. Science. 189(4197):102–114
Fiume L, Manerba M, Vettraino M, Di Stefano G (2014) Inhibition of lactate dehydrogenase activity as an approach to cancer therapy. Future Med Chem 6(4):429–445
Yang Y, Su D, Zhao L, Zhang D, Xu J, Wan J et al (2014) Different effects of LDH-A inhibition by oxamate in non-small cell lung cancer cells. Oncotarget 5(23):11886–11896
Mirebeau-Prunier D, Le Pennec S, Jacques C, Fontaine JF, Gueguen N, Boutet-Bouzamondo N et al (2013) Estrogen-related receptor alpha modulates lactate dehydrogenase activity in thyroid tumors. PLoS One. 8(3):e58683
Zhao D, Zou SW, Liu Y, Zhou X, Mo Y, Wang P et al (2013) Lysine-5 acetylation negatively regulates lactate dehydrogenase A and is decreased in pancreatic cancer. Cancer Cell 23(4):464–476
Sun W, Zhang X, Ding X, Li H, Geng M, Xie Z et al (2015) Lactate dehydrogenase B is associated with the response to neoadjuvant chemotherapy in oral squamous cell carcinoma. PLoS One. 10(5):e0125976
Li C, Chen Y, Bai P, Wang J, Liu Z, Wang T et al (2016) LDHB may be a significant predictor of poor prognosis in osteosarcoma. Am J Transl Res 8(11):4831–4843
Kurpińska A, Suraj J, Bonar E, Zakrzewska A, Stojak M, Sternak M et al (2019) Proteomic characterization of early lung response to breast cancer metastasis in mice. Exp Mol Pathol 107:129–140
Xiao X, Huang X, Ye F, Chen B, Song C, Wen J et al (2016) The miR-34a-LDHA axis regulates glucose metabolism and tumor growth in breast cancer. Sci Rep 6:21735
Manerba M, Di Ianni L, Govoni M, Comparone A, Di Stefano G (2018) The activation of lactate dehydrogenase induced by mTOR drives neoplastic change in breast epithelial cells. PLoS One. 13(8):e0202588
Jin L, Chun J, Pan C, Alesi GN, Li D, Magliocca KR et al (2017) Phosphorylation-mediated activation of LDHA promotes cancer cell invasion and tumour metastasis. Oncogene 36(27):3797–3806
Wang ZY, Loo TY, Shen JG, Wang N, Wang DM, Yang DP et al (2012) LDH-A silencing suppresses breast cancer tumorigenicity through induction of oxidative stress mediated mitochondrial pathway apoptosis. Breast Cancer Res Treat 131(3):791–800
Huang X, Li X, Xie X, Ye F, Chen B, Song C et al (2016) High expressions of LDHA and AMPK as prognostic biomarkers for breast cancer. Breast 30:39–46
Dong T, Liu Z, Xuan Q, Wang Z, Ma W, Zhang Q (2017) Tumor LDH-A expression and serum LDH status are two metabolic predictors for triple negative breast cancer brain metastasis. Sci Rep 7(1):6069
Li L, Kang L, Zhao W, Feng Y, Liu W, Wang T et al (2017) miR-30a-5p suppresses breast tumor growth and metastasis through inhibition of LDHA-mediated Warburg effect. Cancer Lett 400:89–98
McCleland ML, Adler AS, Shang Y, Hunsaker T, Truong T, Peterson D et al (2012) An integrated genomic screen identifies LDHB as an essential gene for triple-negative breast cancer. Cancer Res 72(22):5812–5823
Mack N, Mazzio EA, Bauer D, Flores-Rozas H, Soliman KF (2017) Stable shRNA Silencing of Lactate Dehydrogenase A (LDHA) in human MDA-MB-231 breast cancer cells fails to alter lactic acid production, glycolytic activity. ATP or Survival Anticancer Res 37(3):1205–1212
Pinheiro C, Longatto-Filho A, Azevedo-Silva J, Casal M, Schmitt FC, Baltazar F (2012) Role of monocarboxylate transporters in human cancers: state of the art. J Bioenerg Biomembr 44(1):127–139
Asada K, Miyamoto K, Fukutomi T, Tsuda H, Yagi Y, Wakazono K et al (2003) Reduced expression of GNA11 and silencing of MCT1 in human breast cancers. Oncology 64(4):380–388
Pinheiro C, Reis RM, Ricardo S, Longatto-Filho A, Schmitt F, Baltazar F (2010) Expression of monocarboxylate transporters 1, 2, and 4 in human tumours and their association with CD147 and CD44. J Biomed Biotechnol. 2010:427694
Johnson JM, Cotzia P, Fratamico R, Mikkilineni L, Chen J, Colombo D et al (2017) MCT1 in invasive ductal carcinoma: monocarboxylate metabolism and aggressive breast cancer. Front Cell Dev Biol 5:27
Li KK, Pang JC, Ching AK, Wong CK, Kong X, Wang Y et al (2009) miR-124 is frequently down-regulated in medulloblastoma and is a negative regulator of SLC16A1. Hum Pathol 40(9):1234–1243
Shi P, Chen C, Li X, Wei Z, Liu Z, Liu Y (2019) MicroRNA-124 suppresses cell proliferation and invasion of triple negative breast cancer cells by targeting STAT3. Mol Med Rep 19(5):3667–3675
Romero-Cordoba SL, Rodriguez-Cuevas S, Bautista-Pina V, Maffuz-Aziz A, D’Ippolito E, Cosentino G et al (2018) Loss of function of miR-342-3p results in MCT1 over-expression and contributes to oncogenic metabolic reprogramming in triple negative breast cancer. Sci Rep 8(1):12252
Doyen J, Trastour C, Ettore F et al (2014) Expression of the hypoxia-inducible monocarboxylate transporter MCT4 is increased in triple negative breast cancer and correlates independently with clinical outcome. Biochem Biophys Res Commun 451(1):54–61
Umar SM, Kashyap A, Kahol S, Mathur S, Gogia A, Deo SVS et al (2020) Prognostic and therapeutic relevance of phosphofructokinase platelet-type (PFKP) in breast cancer. Exp Cell Res 10:112282
Kwiatkowska E, Wojtala M, Gajewska A, Soszyński M, Bartosz G, Sadowska-Bartosz I (2016) Effect of 3-bromopyruvate acid on the redox equilibrium in non-invasive MCF-7 and invasive MDA-MB-231 breast cancer cells. J Bioenerg Biomembr 48(1):23–32
Zhang Q, Zhang Y, Zhang P, Chao Z, Xia F, Jiang C et al (2014) Hexokinase II inhibitor, 3-BrPA induced autophagy by stimulating ROS formation in human breast cancer cells. Genes Cancer 5(3–4):100–112
Hou F, Wang H, Zhang Y, Zhu N, Liu H, Li J (2020) Construction and evaluation of folic acid-modified 3-bromopyruvate cubosomes. Med Sci Monit 26:e924620
Yousefi S, Darvishi P, Yousefi Z, Pourfathollah AA (2020) Effect of methyl jasmonate and 3-bromopyruvate combination therapy on mice bearing the 4 T1 breast cancer cell line. J Bioenerg Biomembr 52(2):103–111
Feng X, Wang P, Liu Q, Zhang T, Mai B, Wang X (2015) Glycolytic inhibitors 2-deoxyglucose and 3-bromopyruvate synergize with photodynamic therapy respectively to inhibit cell migration. J Bioenerg Biomembr 47(3):189–197
Wokoun U, Hellriegel M, Emons G, Gründker C (2017) Co-treatment of breast cancer cells with pharmacologic doses of 2-deoxy-D-glucose and metformin: starving tumors. Oncol Rep 37(4):2418–2424
Lucantoni F, Dussmann H, Prehn JHM (2018) Metabolic targeting of breast cancer cells with the 2-Deoxy-D-Glucose and the mitochondrial bioenergetics inhibitor MDIVI-1. Front Cell Dev Biol 6:113
O'Neill S, Porter RK, McNamee N, Martinez VG, O'Driscoll L (2019) 2-Deoxy-D-Glucose inhibits aggressive triple-negative breast cancer cells by targeting glycolysis and the cancer stem cell phenotype. Sci Rep 9(1):3788
Alli E, Solow-Cordero D, Casey SC, Ford JM (2014) Therapeutic targeting of BRCA1-mutated breast cancers with agents that activate DNA repair. Cancer Res 74(21):6205–6215
Nath K, Nelson DS, Heitjan DF, Leeper DB, Zhou R, Glickson JD (2015) Lonidamine induces intracellular tumor acidification and ATP depletion in breast, prostate and ovarian cancer xenografts and potentiates response to doxorubicin. NMR Biomed 28(3):281–290
Goldman A, Khiste S, Freinkman E, Dhawan A, Majumder B, Mondal J et al (2019) Targeting tumor phenotypic plasticity and metabolic remodeling in adaptive cross-drug tolerance. Sci Signal 12(595):eaas8779
Muhammad N, Tan CP, Nawaz U, Wang J, Wang FX, Nasreen S et al (2020) Multiaction platinum(IV) prodrug containing thymidylate synthase inhibitor and metabolic modifier against triple-negative breast cancer. Inorg Chem 59(17):12632–12642
Coelho RG, Calaça Ide C, CelestriniDde M, Correia AH, Costa MA, Sola-Penna M (2011) Clotrimazole disrupts glycolysis in human breast cancer without affecting non-tumoral tissues. Mol Genet Metab 103(4):394-398
Furtado CM, Marcondes MC, Sola-Penna M, de Souza ML, Zancan P (2012) Clotrimazole preferentially inhibits human breast cancer cell proliferation, viability and glycolysis. PLoS One 7(2):e30462
Marcondes MC, Fernandes AC, Itabaiana I Jr, de Souza RO, Sola-Penna M, Zancan P (2015) Nanomicellar formulation of clotrimazole improves its antitumor action toward human breast cancer cells. PLoS One 10(6):e0130555
Clem B, Telang S, Clem A, Yalcin A, Meier J, Simmons A, et al (2008) Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth. Mol Cancer Ther 7(1):110–120
Xintaropoulou C, Ward C, Wise A, Marston H, Turnbull A, Langdon SP (2015) A comparative analysis of inhibitors of the glycolysis pathway in breast and ovarian cancer cell line models. Oncotarget 6(28):25677–25695
Imbert-Fernandez Y, Clem BF, O'Neal J, Kerr DA, Spaulding R, Lanceta L et al (2014) Estradiol stimulates glucose metabolism via 6-phosphofructo-2-kinase (PFKFB3). J Biol Chem 289(13):9440–9448
Gomez LS, Zancan P, Marcondes MC, Ramos-Santos L, Meyer-Fernandes JR, Sola-Penna M et al (2013) Resveratrol decreases breast cancer cell viability and glucose metabolism by inhibiting 6-phosphofructo-1-kinase. Biochimie 95(6):1336–1343
Siddiqui FA, Prakasam G, Chattopadhyay S, Rehman AU, Padder RA, Ansari MA, et al (2018) Curcumin decreases Warburg effect in cancer cells by down-regulating pyruvate kinase M2 via mTOR-HIF1α inhibition. Sci Rep 8(1):8323
Silvestri A, Palumbo F, Rasi I, Posca D, Pavlidou T, Paoluzi S, et al (2015) Metformin induces apoptosis and downregulates pyruvate kinase M2 in breast cancer cells only when grown in nutrient-poor conditions. PLoS One 10(8):e0136250
Wahdan-Alaswad RS, Edgerton SM, Salem HS, Thor AD (2018) Metformin targets glucose metabolism in triple negative breast cancer. J Oncol Transl Res 4(1):129
Guan M, Tong Y, Guan M, Liu X, Wang M, Niu R et al (2018) Lapatinib inhibits breast cancer cell proliferation by influencing PKM2 expression. Technol Cancer Res Treat 17:1533034617749418
Chen J, Xie J, Jiang Z, Wang B, Wang Y, Hu X (2011) Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2. Oncogene 30(42):4297–4306
Kéri G, Erchegyi J, Horváth A, Mezõ I, Idei M, Vántus T et al (1996) A tumor-selective somatostatin analog (TT-232) with strong in vitro and in vivo antitumor activity. Proc Natl Acad Sci USA 93(22):12513–12518
Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM et al (2010) Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci USA 107(5):2037–2042
Farabegoli F, Vettraino M, Manerba M, Fiume L, Roberti M, Di Stefano G (2012) Galloflavin, a new lactate dehydrogenase inhibitor, induces the death of human breast cancer cells with different glycolytic attitude by affecting distinct signaling pathways. Eur J Pharm Sci 47(4):729–738
Van Poznak C, Seidman AD, Reidenberg MM, Moasser MM, Sklarin N, Van Zee K et al (2001) Oral gossypol in the treatment of patients with refractory metastatic breast cancer: a phase I/II clinical trial. Breast Cancer Res Treat 66(3):239–248
Thornburg JM, Nelson KK, Clem BF, Lane AN, Arumugam S, Simmons A et al (2008) Targeting aspartate aminotransferase in breast cancer. Breast Cancer Res 10(5):R84
Zhou M, Zhao Y, Ding Y, Liu H, Liu Z, Fodstad O et al (2010) Warburg effect in chemosensitivity: targeting lactate dehydrogenase-A re-sensitizes taxol-resistant cancer cells to taxol. Mol Cancer 9:33
Cui B, Luo Y, Tian P, Peng F, Lu J, Yang Y et al (2019) Stress-induced epinephrine enhances lactate dehydrogenase A and promotes breast cancer stem-like cells. J Clin Invest 129(3):1030–1046
Guan X, Bryniarski MA, Morris ME (2018) In vitro and in vivo efficacy of the monocarboxylate transporter 1 inhibitor AR-C155858 in the murine 4T1 breast cancer tumor model. AAPS J 21(1):3
Andersen AP, Flinck M, Oernbo EK, Pedersen NB, Viuff BM, Pedersen SF (2016) Roles of acid-extruding ion transporters in regulation of breast cancer cell growth in a 3-dimensional microenvironment. Mol Cancer 15(1):45
Jonnalagadda S, Jonnalagadda SK, Ronayne CT, Nelson GL, Solano LN, Rumbley J et al (2019) Novel N,N-dialkylcyanocinnamic acids as monocarboxylate transporter 1 and 4 inhibitors. Oncotarget 10(24):2355–2368
Morais-Santos F, Miranda-Gonçalves V, Pinheiro S, Vieira AF, Paredes J, Schmitt FC et al (2013) Differential sensitivities to lactate transport inhibitors of breast cancer cell lines. Endocr Relat Cancer 21(1):27–38
Azevedo C, Correia-Branco A, Araújo JR, Guimarães JT, Keating E, Martel F (2015) The chemopreventive effect of the dietary compound kaempferol on the MCF-7 human breast cancer cell line is dependent on inhibition of glucose cellular uptake. Nutr Cancer 67(3):504–513
Funding
The work was supported by the DST SERB ECR grant (No.2017/001836), and AIIMS Intramural grants (No. A-515).
Author information
Authors and Affiliations
Contributions
CPP proposed the initial idea. ADJR & CPP analyzed the literature, designed and formatted the review. SRM, AG, SVSD & PM provided inputs and involved in critical revision.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arundhathi, J.R.D., Mathur, S., Gogia, A. et al. Metabolic changes in triple negative breast cancer-focus on aerobic glycolysis. Mol Biol Rep 48, 4733–4745 (2021). https://doi.org/10.1007/s11033-021-06414-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06414-w