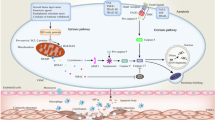
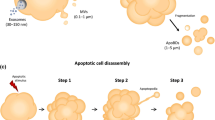
Abstract
In a mature organism, tissue homeostasis is regulated by cell division and cell demise as the two major physiological procedures. There is increasing evidence that deregulation of these processes is important in the pathogenicity of main diseases, including myocardial infarction, stroke, atherosclerosis, and inflammatory diseases. Therefore, there are ongoing efforts to discover modulating factors of the cell cycle and cell demise planners aiming at shaping innovative therapeutically modalities to the therapy of such diseases. Although the life of a cell is terminated by several modes of action, a few cell deaths exist—some of which resemble apoptosis and/or necrosis, and most of them are different from one another—that contribute to a wide range of functions to either support or disrupt the homoeostasis. Even in normal physiological conditions, cell life is severe within the cardiovascular system. Cells are persistently undergoing stretch, contraction, injurious metabolic byproducts, and hemodynamic forces, and a few of cells sustain decade-long lifetimes. The duration of vascular disease causes further exposure of vascular cells to a novel range of offences, most of which induce cell death. There is growing evidence on consequences of direct damage to a cell, as well as on responses of adjacent and infiltrating cells, which also have an effect on the pathology. In this study, by focusing on different pathways of cell death in different vascular diseases, an attempt is made to open a new perspective on the therapeutic goals associated with cell death in these diseases.





Similar content being viewed by others
Data availability
Exist.
Abbreviations
- NO:
-
Nitric Oxide
- GMP:
-
Guanosine monophosphate
- SMC:
-
Smooth muscle cells
- TUNEL:
-
Transferase dUTP nick end labeling
- ISEL:
-
In situ nick translation
- Bcl‐2:
-
B-cell lymphoma 2
- DAMPs:
-
Danger-associated molecular patterns
- NETs:
-
Neutrophil extracellular traps
- MPO:
-
Myeloperoxidase
- RIPK1:
-
Receptor-interacting protein kinase-1
- TRAFF2/5:
-
TNF receptor-associated factor 2/5
- TRADD:
-
Type 1–associated death domain
- PAH:
-
Pulmonary arterial hypertension
- TLR:
-
Toll‐like receptor
- NLR:
-
Nod‐like receptor
- DAMPs:
-
Damage‐associated molecular patterns
- RIPK3:
-
Receptor-interacting serine/threonine-protein kinase 3
- IR:
-
Ischemia–reperfusion
- PGAM5:
-
PGAM Family Member 5
- NLRP3:
-
NLR family pyrin domain containing 3
- HMGB1:
-
For high-mobility group box 1
- PDGF:
-
Platelet-derived growth factor
- TGF-β:
-
Transforming growth factor beta
- VCAM-1:
-
Vascular cell adhesion protein 1
- DAMP:
-
Damage-associated molecular patterns
- CVD:
-
Cardiovascular disease
- GPX4:
-
Glutathione peroxidase 4
- VSMCs:
-
Vascular smooth muscle cells
- VCI:
-
Vascular cognitive impairment
- AD:
-
Alzheimer’s disease
- LDCD:
-
Lysosome-dependent cell death
- OxLDL:
-
Oxidized low-density lipoprotein (oxLDL)
- ROS:
-
Oxygen species
- LC3:
-
Protein 1A/1B-light chain 3
References
Nirmala JG, Lopus M (2020) Cell death mechanisms in eukaryotes. Cell Biol Toxicol 36(2):145–164
Hyman BT, Yuan J (2012) Apoptotic and non-apoptotic roles of caspases in neuronal physiology and pathophysiology. Nat Rev Neurosci 13(6):395–406
Wlodkowic D, Telford W, Skommer J, Darzynkiewicz Z (2011) Apoptosis and beyond: cytometry in studies of programmed cell death. Methods in cell biology: 103. Elsevier, London, pp 55–98
Zhu C, Wang X, Xu F, Bahr B, Shibata M, Uchiyama Y et al (2005) The influence of age on apoptotic and other mechanisms of cell death after cerebral hypoxia–ischemia. Cell Death Differ 12(2):162–176
Berber P et al (2017) An eye on age-related macular degeneration: the role of microRNAs in disease pathology. Mol Diagn Ther 21(1):31–43
MacMicking J, Xie Q-W, Nathan C (1997) Nitric oxide and macrophage function. Ann Rev Immunol 15(1):323–50
Das A, Smolenski A, Lohmann SM, Kukreja RC (2006) Cyclic GMP-dependent protein kinase Iα attenuates necrosis and apoptosis following ischemia/reoxygenation in adult cardiomyocyte. J Biol Chem 281(50):38644–38652
Beck K-F, Eberhardt W, Frank S, Huwiler A, Messmer U, Muhl H et al (1999) Inducible NO synthase: role in cellular signalling. J Exp Biol 202(6):645–653
Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G et al (2013) The vascular endothelium and human diseases. Int J Biol Sci 9(10):1057
Huang YQ et al (2016) The relationship of plasma miR-29a and oxidized low density lipoprotein with atherosclerosis. Cell Physiol and Biochem 40(6):1521–1528
Burnstock G (2002) Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol 22(3):364–373
Weber C, Schober A, Zernecke A (2004) Chemokines: key regulators of mononuclear cell recruitment in atherosclerotic vascular disease. Arterioscler Thromb Vasc Biol 24(11):1997–2008
Davidson SM, Duchen MR (2007) Endothelial mitochondria: contributing to vascular function and disease. Circ Res 100(8):1128–1141
Kim H, Blanco F (2007) Cell death and apoptosis in ostearthritic cartilage. Curr Drug Targets 8(2):333–345
Mallat Z, Tedgui A (2000) Apoptosis in the vasculature: mechanisms and functional importance. Br J Pharmacol 130(5):947–962
Hamet P, deBlois D, Dam T-V, Richard L, Teiger E, Tea B-S et al (1996) Apoptosis and vascular wall remodeling in hypertension. Can J Physiol Pharmacol 74(7):850–861
Wang K (2015) Autophagy and apoptosis in liver injury. Cell Cycle (Georgetown, Tex) 14(11):1631–1642
Kyrylkova K, Kyryachenko S, Leid M, Kioussi C (2012) Detection of apoptosis by TUNEL assay. Methods Mol Biol (Clifton, NJ) 887:41–47
Kockx MM (1998) Apoptosis in the atherosclerotic plaque: quantitative and qualitative aspects. Arterioscler Thromb Vasc Biol 18(10):1519–1522
Safar M, Blacher J, Mourad J, London G (2000) Stiffness of carotid artery wall material and blood pressure in humans: application to antihypertensive therapy and stroke prevention. Stroke 31(3):782–790
Pleouras DS, Sakellarios AI, Tsompou P, Kigka V, Kyriakidis S, Rocchiccioli S et al (2020) Simulation of atherosclerotic plaque growth using computational biomechanics and patient-specific data. Sci Rep 10(1):1–14
Mackie EJ, Scott-Burden T, Hahn A, Kern F, Bernhardt J, Regenass S et al (1992) Expression of tenascin by vascular smooth muscle cells. Alterations in hypertensive rats and stimulation by angiotensin II. Am J Pathol 141(2):377
Shi Y-X, Chen Y, Zhu Y-Z, Huang G-Y, Moore PK, Huang S-H et al (2007) Chronic sodium hydrosulfide treatment decreases medial thickening of intramyocardial coronary arterioles, interstitial fibrosis, and ROS production in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 293(4):H2093–H2100
Yang HL, Korivi M, Chen CH, Peng WJ, Chen CS, Li ML et al (2017) A ntrodia camphorata attenuates cigarette smoke-induced ROS production, DNA damage, apoptosis, and inflammation in vascular smooth muscle cells, and atherosclerosis in ApoE-deficient mice. Environ Toxicol 32(8):2070–2084
Fortuño MA, Ravassa S, Etayo JC, Díez J (1998) Overexpression of bax protein and enhanced apoptosis in the left ventricle of spontaneously hypertensive rats: effects of AT1 blockade with losartan. Hypertension 32(2):280–6
Morales-Cano D, Calviño E, Rubio V, Herráez A, Sancho P, Tejedor MC et al (2013) Apoptosis induced by paclitaxel via Bcl-2, Bax and caspases 3 and 9 activation in NB4 human leukaemia cells is not modulated by ERK inhibition. Exp Toxicol Pathol 65(7–8):1101–1108
Li W, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y et al (2007) Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells 25(8):2118–2127
Durand E, Mallat Z, Addad F, Vilde F, Desnos M, Guérot C et al (2002) Time courses of apoptosis and cell proliferation and their relationship to arterial remodeling and restenosis after angioplasty in an atherosclerotic rabbit model. J Am Coll Cardiol 39(10):1680–1685
Perlman H, Maillard L, Krasinski K, Walsh K (1997) Evidence for the rapid onset of apoptosis in medial smooth muscle cells after balloon injury. Circulation 95(4):981–987
Pollman MJ, Hall JL, Gibbons GH (1999) Determinants of vascular smooth muscle cell apoptosis after balloon angioplasty injury: influence of redox state and cell phenotype. Circ Res 84(1):113–121
Majesky MW, Horita H, Ostriker A, Lu S, Regan JN, Bagchi A et al (2017) Differentiated smooth muscle cells generate a subpopulation of resident vascular progenitor cells in the adventitia regulated by Klf4. Circ Res 120(2):296–311
Burtea C, Laurent S, Lancelot E, Ballet S, Murariu O, Rousseaux O et al (2009) Peptidic targeting of phosphatidylserine for the MRI detection of apoptosis in atherosclerotic plaques. Mol Pharm 6(6):1903–1919
Henson PM, Hume DA (2006) Apoptotic cell removal in development and tissue homeostasis. Trends Immunol 27(5):244–250
Thorp EB (2010) Mechanisms of failed apoptotic cell clearance by phagocyte subsets in cardiovascular disease. Apoptosis 15(9):1124–1136
Johnson JL, Newby AC (2009) Macrophage heterogeneity in atherosclerotic plaques. Curr Opin Lipidol 20(5):370
Coornaert I, Hofmans S, Devisscher L, Augustyns K, Van Der Veken P, De Meyer GR et al (2018) Novel drug discovery strategies for atherosclerosis that target necrosis and necroptosis. Expert Opin Drug Discov 13(6):477–488
Proskuryakov SY, Konoplyannikov AG, Gabai VL (2003) Necrosis: a specific form of programmed cell death? Exp Cell Res 283(1):1–16
Kim EH, Wong S-W, Martinez J (2019) Programmed necrosis and disease: we interrupt your regular programming to bring you necroinflammation. Cell Death Differ 26(1):25–40
Kolb JP, Oguin TH III, Oberst A, Martinez J (2017) Programmed cell death and inflammation: winter is coming. Trends Immunol 38(10):705–718
Sun L, Wang H, Wang Z, He S, Chen S, Liao D et al (2012) Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148(1–2):213–227
Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD et al (2010) Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA 107(36):15880–15885
Jiménez-Alcázar M, Rangaswamy C, Panda R, Bitterling J, Simsek YJ, Long AT et al (2017) Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science 358(6367):1202–1206
Söderberg D, Segelmark M (2016) Neutrophil extracellular traps in ANCA-associated vasculitis. Front Immunol 7:256
Döring Y, Soehnlein O, Weber C (2014) Neutrophils cast NETs in atherosclerosis: employing peptidylarginine deiminase as a therapeutic target. Am Heart Assoc. https://doi.org/10.1161/CIRCRESAHA.114.303479
Josefs T, Barrett TJ, Brown EJ, Quezada A, Wu X, Voisin M et al (2020) Neutrophil extracellular traps (NETs) promote macrophage inflammation and impair atherosclerosis resolution in mice with diabetes. JCI Insight. https://doi.org/10.1172/jci.insight.134796
Megens RT, Vijayan S, Lievens D, Doering Y, van Zandvoort MA, Grommes J et al (2012) Presence of luminal neutrophil extracellular traps in atherosclerosis. Thromb Haemost 107(03):597–598
Knight JS, Luo W, O’Dell AA, Yalavarthi S, Zhao W, Subramanian V et al (2014) Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ Res 114(6):947–956
Saha P, San Yeoh B, Xiao X, Golonka RM, Singh V, Wang Y et al (2019) PAD4-dependent NETs generation are indispensable for intestinal clearance of Citrobacter rodentium. Mucosal Immunol 12(3):761–771
Borissoff JI, Joosen IA, Versteylen MO, Brill A, Fuchs TA, Savchenko AS et al (2013) Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler Thromb Vasc Biol 33(8):2032–2040
Kambas K, Mitroulis I, Ritis K (2012) The emerging role of neutrophils in thrombosis—the journey of TF through NETs. Front Immunol 3:385
Fuentes QE, Fuentes QF, Andrés V, Pello OM, de Mora JF, Palomo GI (2013) Role of platelets as mediators that link inflammation and thrombosis in atherosclerosis. Platelets 24(4):255–262
Darbousset R, Thomas GM, Mezouar S, Frere C, Bonier R, Mackman N et al (2012) Tissue factor–positive neutrophils bind to injured endothelial wall and initiate thrombus formation. Blood 120(10):2133–2143
Jennette JC, Falk RJ, Hu P, Xiao H (2013) Pathogenesis of antineutrophil cytoplasmic autoantibody–associated small-vessel vasculitis. Annu Rev Pathol 8:139–160
Ni H-M, Chao X, Kaseff J, Deng F, Wang S, Shi Y-H et al (2019) Receptor-interacting serine/threonine-protein kinase 3 (RIPK3)–mixed lineage kinase domain-like protein (mlkl)–mediated necroptosis contributes to ischemia-reperfusion injury of steatotic livers. Am J Pathol 189(7):1363–1374
Luedde T, Kaplowitz N, Schwabe RF (2014) Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology 147(4):765-783.e4
Linkermann A, Green DR (2014) Necroptosis. N Engl J Med 370(5):455–465
Xiao G, Zhuang W, Wang T, Lian G, Luo L, Ye C et al (2020) Transcriptomic analysis identifies toll-like and nod-like pathways and necroptosis in pulmonary arterial hypertension. J Cell Mol Med 24(19):11409–11421
Xiao G, Zhuang W, Wang T, Luo L, Ye C, Wang H et al (2020) Transcriptomic analysis identifies necroptosis and toll-like receptor pathways in pulmonary arterial hypertension. SSRN. https://doi.org/10.2139/ssrn.3544807
Li C, Ma Q, Toan S, Wang J, Zhou H, Liang J (2020) SERCA overexpression reduces reperfusion-mediated cardiac microvascular damage through inhibition of the calcium/MCU/mPTP/necroptosis signaling pathways. Redox Biol 36:101659
Zhou H, Li D, Zhu P, Ma Q, Toan S, Wang J et al (2018) Inhibitory effect of melatonin on necroptosis via repressing the Ripk3-PGAM5-CypD-mPTP pathway attenuates cardiac microvascular ischemia–reperfusion injury. J Pineal Res 65(3):e12503
Leeper NJ (2016) The role of necroptosis in atherosclerotic disease. JACC 1(6):548–550
Khalili M, Radosevich JA (2018) Pyronecrosis. In: Radosevich J (ed) Apoptosis and beyond: the many ways cells die. Wiley, Hoboken, pp 225–236
Pasparakis M, Vandenabeele P (2015) Necroptosis and its role in inflammation. Nature 517(7534):311–320
Edwan J, Tran T, Abu-Asab M, Goldbach-Mansky R, Colbert R (2012) STAT3 plays a central role in NLRP3 inflammasome-mediated IL-1β production and pyronecrosis: 310. Arthr Rheum 64:S136
Xu Y-J, Zheng L, Hu Y-W, Wang Q (2018) Pyroptosis and its relationship to atherosclerosis. Clin Chim Acta 476:28–37
Wu X, Zhang H, Qi W, Zhang Y, Li J, Li Z et al (2018) Nicotine promotes atherosclerosis via ROS-NLRP3-mediated endothelial cell pyroptosis. Cell Death Dis 9(2):1–12
Jia C, Chen H, Zhang J, Zhou K, Zhuge Y, Niu C et al (2019) Role of pyroptosis in cardiovascular diseases. Int Immunopharmacol 67:311–318
Yang J-R, Yao F-H, Zhang J-G, Ji Z-Y, Li K-L, Zhan J et al (2014) Ischemia-reperfusion induces renal tubule pyroptosis via the CHOP-caspase-11 pathway. Am J Physiol Renal Physiol 306(1):F75–F84
Toldo S, Mauro AG, Cutter Z, Abbate A (2018) Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 315(6):H1553–H1568
Lu F, Lan Z, Xin Z, He C, Guo Z, Xia X et al (2020) Emerging insights into molecular mechanisms underlying pyroptosis and functions of inflammasomes in diseases. J Cell Physiol 235(4):3207–3221
Bergsbaken T, Fink SL, Cookson BT (2009) Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7(2):99–109
Krishna S, Overholtzer M (2016) Mechanisms and consequences of entosis. Cell Mol Life Sci 73(11–12):2379–2386
Zeng C, Zeng B, Dong C, Liu J, Xing F (2020) Rho-ROCK signaling mediates entotic cell death in tumor. Cell Death Discov 6:4
Florey O, Krajcovic M, Sun Q, Overholtzer M (2010) Entosis. Curr Biol 20(3):R88–R89
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X et al (2016) Ferroptosis: process and function. Cell Death Differ 23(3):369–379
Battaglia AM, Chirillo R, Aversa I, Sacco A, Costanzo F, Biamonte F (2020) Ferroptosis and cancer: mitochondria meet the “iron maiden” cell death. Cells 9(6):1505
Zhou B, Liu J, Kang R, Klionsky DJ, Kroemer G, Tang D (eds) (2019) Ferroptosis is a type of autophagy-dependent cell death, Seminars in cancer biology. Elsevier, London
Čepelak I, Dodig S, Čepelak DD (2020) Ferroptosis: regulated cell death. Arhiv za higijenu rada i toksikologiju 71(2):99–109
Hu Z, Zhang H, Yang SK, Wu X, He D, Cao K et al (2019) Emerging role of ferroptosis in acute kidney injury. Oxid Med Cell Longev 2019:8010614
Du J, Wang T, Li Y, Zhou Y, Wang X, Yu X et al (2019) DHA inhibits proliferation and induces ferroptosis of leukemia cells through autophagy dependent degradation of ferritin. Free Radical Biol Med 131:356–369
Sampilvanjil A, Karasawa T, Yamada N, Komada T, Higashi T, Baatarjav C et al (2020) Cigarette smoke extract induces ferroptosis in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 318(3):H508–H518
Land WG (2020) Damage-associated molecular patterns in human diseases: danger signals as diagnostics, prognostics, and therapeutic targets, vol 2. Springer Nature, Switzerland
Wang Q (2015) The role of smooth muscle cell death in vascular inflammation and abdominal aortic aneurysm. The University of Wisconsin-Madison, Madison
Yan N, Zhang J-J (2019) The emerging roles of ferroptosis in vascular cognitive impairment. Front Neurosci 13:811
Bowler J, Hachinski V (1995) Vascular cognitive impairment: a new approach to vascular dementia. Bailliere’s Clin Neurol 4(2):357–376
Wang F, Salvati A, Boya P (2018) Lysosome-dependent cell death and deregulated autophagy induced by amine-modified polystyrene nanoparticles. Open Biol 8(4):170271
Appelqvist H, Sandin L, Björnström K, Saftig P, Garner B, Öllinger K et al (2012) Sensitivity to lysosome-dependent cell death is directly regulated by lysosomal cholesterol content. PLoS ONE 7(11):e50262
Yu F, Chen Z, Wang B, Jin Z, Hou Y, Ma S et al (2016) The role of lysosome in cell death regulation. Tumor Biol 37(2):1427–1436
Nussenzweig SC, Verma S, Finkel T (2015) The role of autophagy in vascular biology. Circ Res 116(3):480–488
Signorelli P, Avagliano L, Virgili E, Gagliostro V, Doi P, Braidotti P et al (2011) Autophagy in term normal human placentas. Placenta 32(6):482–485
De Munck DG, Leloup AJ, De Meyer GR, Martinet W, Fransen P (2020) Defective autophagy in vascular smooth muscle cells increases passive stiffness of the mouse aortic vessel wall. Pflugers Archiv. https://doi.org/10.1007/s00424-020-02408-y
Osonoi Y, Mita T, Azuma K, Nakajima K, Masuyama A, Goto H et al (2018) Defective autophagy in vascular smooth muscle cells enhances cell death and atherosclerosis. Autophagy 14(11):1991–2006
Martinet W, De Meyer GR (2009) Autophagy in atherosclerosis: a cell survival and death phenomenon with therapeutic potential. Circ Res 104(3):304–317
Ni H, Xu S, Chen H, Dai Q (2020) Nicotine modulates CTSS (Cathepsin S) synthesis and secretion through regulating the autophagy-lysosomal machinery in atherosclerosis. Arterioscler Thromb Vasc Biol 40(9):2054–2069
Li X, Zhou Y, Zhang X, Cao X, Wu C, Guo P (2017) Cordycepin stimulates autophagy in macrophages and prevents atherosclerotic plaque formation in ApoE-/-mice. Oncotarget 8(55):94726
Wu H, Ploeger JM, Kamarajugadda S, Mashek DG, Mashek MT, Manivel JC et al (2019) Evidence for a novel regulatory interaction involving cyclin D1, lipid droplets, lipolysis, and cell cycle progression in hepatocytes. Hepatol Commun 3(3):406–422
Chan LL-Y, Shen D, Wilkinson AR, Patton W, Lai N, Chan E et al (2012) A novel image-based cytometry method for autophagy detection in living cells. Autophagy. 8(9):1371–1382
Wang L, Liu S, Pan B, Cai H, Zhou H, Yang P et al (2020) The role of autophagy in abdominal aortic aneurysm: protective but dysfunctional. Cell Cycle. https://doi.org/10.1080/15384101.2020.1823731
Ramadan A, Al-Omran M, Verma S (2017) The putative role of autophagy in the pathogenesis of abdominal aortic aneurysms. Atherosclerosis 257:288–296
Salmon M, Spinosa M, Zehner ZE, Upchurch GR, Ailawadi G (2019) Klf4, Klf2, and Zfp148 activate autophagy-related genes in smooth muscle cells during aortic aneurysm formation. Physiol Rep 7(8):e14058
Wu Q-Y, Cheng Z, Zhou Y-Z, Zhao Y, Li J-M, Zhou X-M et al (2020) A novel STAT3 inhibitor attenuates angiotensin II-induced abdominal aortic aneurysm progression in mice through modulating vascular inflammation and autophagy. Cell Death Dis 11(2):1–16
LaRocca TJ, Gioscia-Ryan RA, Hearon CM Jr, Seals DR (2013) The autophagy enhancer spermidine reverses arterial aging. Mech Ageing Dev 134(7–8):314–320
McCarthy CG, Wenceslau CF, Calmasini FB, Klee NS, Brands MW, Joe B et al (2019) Reconstitution of autophagy ameliorates vascular function and arterial stiffening in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 317(5):H1013–H1027
Li Z, Wang J, Yang X (2015) Functions of autophagy in pathological cardiac hypertrophy. Int J Biol Sci 11(6):672
Sahani MH, Itakura E, Mizushima N (2014) Expression of the autophagy substrate SQSTM1/p62 is restored during prolonged starvation depending on transcriptional upregulation and autophagy-derived amino acids. Autophagy 10(3):431–441
Odagiri S, Tanji K, Mori F, Kakita A, Takahashi H, Wakabayashi K (2012) Autophagic adapter protein NBR1 is localized in lewy bodies and glial cytoplasmic inclusions and is involved in aggregate formation in α-synucleinopathy. Acta Neuropathol 124(2):173–186
Liu Y, Levine B (2015) Autosis and autophagic cell death: the dark side of autophagy. Cell Death Differ 22(3):367–376
Liu Y, Shoji-Kawata S, Sumpter RM, Wei Y, Ginet V, Zhang L et al (2013) Autosis is a Na+, K+-ATPase–regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia–ischemia. Proc Natl Acad Sci USA 110(51):20364–20371
Robinson N, Ganesan R, Hegedűs C, Kovács K, Kufer TA, Virág L (2019) Programmed necrotic cell death of macrophages: focus on pyroptosis, necroptosis, and parthanatos. Redox Biol 26:101239
Fatokun AA, Dawson VL, Dawson TM (2014) Parthanatos: mitochondrial-linked mechanisms and therapeutic opportunities. Br J Pharmacol 171(8):2000–2016
Feldman CC, Zhou T, Phan N, Liu B (2017) Parthanatos is involved in hydrogen peroxide induced vascular smooth muscle cell death. Arterioscler Thromb Vasc Biol 37(suppl 1):A239
Xu S, Bai P, Little PJ, Liu P (2014) Poly (ADP-ribose) polymerase 1 (PARP1) in atherosclerosis: from molecular mechanisms to therapeutic implications. Med Res Rev 34(3):644–675
Lemaire-Ewing S, Prunet C, Montange T, Vejux A, Berthier A, Bessede G et al (2005) Comparison of the cytotoxic, pro-oxidant and pro-inflammatory characteristics of different oxysterols. Cell Biol Toxicol 21(2):97–114
Björkhem I, Diczfalusy U (2020) Side-chain oxidized oxysterols in health and disease. In: Rozman D, Gebhardt R (eds) Mammalian sterols. Springer, Cham, pp 41–79
Nury T, Yammine A, Ghzaiel I, Sassi K, Zarrouk A, Brahmi F et al (2021) Attenuation of 7-ketocholesterol-and 7β-hydroxycholesterol-induced oxiapoptophagy by nutrients, synthetic molecules and oils: potential for the prevention of age-related diseases. Ageing Res Rev 68:101324
Brown AJ, Jessup W (1999) Oxysterols and atherosclerosis. Atherosclerosis 142(1):1–28
Shibata N, Glass CK (2010) Macrophages, oxysterols and atherosclerosis. Circ J. https://doi.org/10.1253/circj.cj-10-0860
Brown RB (2019) Phospholipid packing defects and oxysterols in atherosclerosis: dietary prevention and the French paradox. Biochimie 167:145–151
Zarrouk A, Vejux A, Mackrill J, O’Callaghan Y, Hammami M, O’Brien N et al (2014) Involvement of oxysterols in age-related diseases and ageing processes. Ageing Res Rev 18:148–162
Vurusaner B, Leonarduzzi G, Gamba P, Poli G, Basaga H (2016) Oxysterols and mechanisms of survival signaling. Mol Aspects Med 49:8–22
De Saint-Hubert M, Prinsen K, Mortelmans L, Verbruggen A, Mottaghy FM (2009) Molecular imaging of cell death. Methods 48(2):178–187
Loo DT (2011) In situ detection of apoptosis by the TUNEL assay: an overview of techniques. In: Didenko VV (ed) DNA damage detection in situ, ex vivo, and in vivo. Humana Press, Totowa, pp 3–13
Lee K, Cavanaugh L, Leung H, Yan F, Ahmadi Z, Chong B et al (2018) Quantification of NETs-associated markers by flow cytometry and serum assays in patients with thrombosis and sepsis. Int J Lab Hematol 40(4):392–399
Cai Z, Jitkaew S, Zhao J, Chiang H-C, Choksi S, Liu J et al (2014) Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol 16(1):55–65
Bell CW, Jiang W, Reich CF III, Pisetsky DS (2006) The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol 291(6):C1318–C1325
Wu Y, Song X, Wang N, Cong S, Zhao X, Rai R et al (2020) Carbon dots from roasted chicken accumulate in lysosomes and induce lysosome-dependent cell death. Food Funct 11(11):10105–10113
Tasdemir E, Galluzzi L, Maiuri MC, Criollo A, Vitale I, Hangen E et al (2008) Methods for assessing autophagy and autophagic cell death. In: Deretic V (ed) Autophagosome and phagosome. Humana Press, Totowa, pp 29–76
Dong W, Yang B, Wang Y, Yuan J, Fan Y, Song E et al (2018) Polybrominated diphenyl ethers quinone induced parthanatos-like cell death through a reactive oxygen species-associated poly (ADP-ribose) polymerase 1 signaling. Chem Res Toxicol 31(11):1164–1171
Nury T, Zarrouk A, Yammine A, Mackrill JJ, Vejux A, Lizard G (2020) Oxiapoptophagy: a type of cell death induced by some oxysterols. Br J Pharmacol. https://doi.org/10.1111/bph.15173
Gibbons GH, Dzau VJ (1996) Molecular therapies for vascular diseases. Science 272(5262):689–693
Shroff R, Long DA, Shanahan C (2013) Mechanistic insights into vascular calcification in CKD. J Am Soc Nephrol 24(2):179–189
Pollman MJ, Hall JL, Mann MJ, Zhang L, Gibbons GH (1998) Inhibition of neointimal cell bcl-x expression induces apoptosis and regression of vascular disease. Nat Med 4(2):222–227
Sata M, Suhara T, Walsh K (2000) Vascular endothelial cells and smooth muscle cells differ in expression of fas and fas ligand and in sensitivity to fas ligand–induced cell death: implications for vascular disease and therapy. Arterioscler Thromb Vasc Biol 20(2):309–316
Choy JC, Kerjner A, Wong BW, McManus BM, Granville DJ (2004) Perforin mediates endothelial cell death and resultant transplant vascular disease in cardiac allografts. Am J Pathol 165(1):127–133
Ng EW, Shima DT, Calias P, Cunningham ET, Guyer DR, Adamis AP (2006) Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discovery 5(2):123–132
Liu X, Cheng Y, Yang J, Krall TJ, Huo Y, Zhang C (2010) An essential role of PDCD4 in vascular smooth muscle cell apoptosis and proliferation: implications for vascular disease. Am J Physiol Cell Physiol 298(6):C1481–C1488
Pamukcu B, Lip GY, Shantsila E (2011) The nuclear factor–kappa B pathway in atherosclerosis: a potential therapeutic target for atherothrombotic vascular disease. Thromb Res 128(2):117–123
Lovren F, Pan Y, Quan A, Singh KK, Shukla PC, Gupta N et al (2012) MicroRNA-145 targeted therapy reduces atherosclerosis. Circulation 126(11 suppl 1):S81–S90
Kang DH, Kang SW (2013) Targeting cellular antioxidant enzymes for treating atherosclerotic vascular disease. Biomol Ther 21(2):89
Salabei JK, Cummins TD, Singh M, Jones SP, Bhatnagar A, Hill BG (2013) PDGF-mediated autophagy regulates vascular smooth muscle cell phenotype and resistance to oxidative stress. Biochem J 451(3):375–388
Heath JM, Sun Y, Yuan K, Bradley WE, Litovsky S, Dell’Italia LJ et al (2014) Activation of AKT by O-linked N-acetylglucosamine induces vascular calcification in diabetes mellitus. Circ Res 114(7):1094–1102
Phadwal K, Feng D, Zhu D, MacRae VE (2020) Autophagy as a novel therapeutic target in vascular calcification. Pharmacol Ther 206:107430
Funding
None.
Author information
Authors and Affiliations
Contributions
SS: Data gathering, writing draft. Ak: Editing manuscript. MHSM: Scientific editor. MA: Design figures.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest to disclose.
Consent to participate
All authors consent to patriciate.
Consent to publication
All authors consent to publish.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saberianpour, S., Karimi, A., Saeed modaghegh, M.H. et al. Different types of cell death in vascular diseases. Mol Biol Rep 48, 4687–4702 (2021). https://doi.org/10.1007/s11033-021-06402-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06402-0