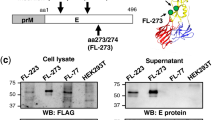
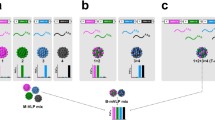
Abstract
Dengue virus and Zika virus are arthropod-borne flaviviruses that cause millions of infections worldwide. The co-circulation of both viruses makes serological diagnosis difficult as they share high amino acid similarities in viral proteins. Antigens are one of the key reagents in the differential diagnosis of these viruses through the detection of IgG antibodies in serological assays during the convalescent-phase of infections. Here, we report the expression of Dengue virus (DENV) and Zika virus (ZIKV) antigens containing non-conserved and immunodominant amino acid sequences using the baculovirus expression vector system in insect cells. We designed DENV and ZIKV antigens based on the domain III of the E protein (EDIII) after analyzing previously reported epitopes and by multiple alignment of the most important flaviviruses. The ZIKV and DENV multi-epitope genes were designed as tandem repeats or impaired repeats separated by tetra- or hexa-glycine linkers. The biochemical analyses revealed adequate expression of the antigens. Then, the obtained multi-epitope antigens were semi-purified in a sucrose gradient and tested using patients’ sera collected during the convalescent-phase that were previously diagnosed positive for anti-DENV and -ZIKV IgG antibodies. The optimal serum dilution was 1:200, and the mean absorbance values in the preliminary tests show that multi-epitope antigens have been recognized by human sera. The production of both antigens using the multi-epitope strategy in the eukaryotic system and based on the EDIII regions provide a proof of concept for the use of antigens in the differentiation between DENV and ZIKV.




Similar content being viewed by others
References
Hasan SS, Sevvana M, Kuhn RJ, Rossmann MG (2018) Structural biology of zika virus and other flaviviruses. Nat Struct Mol Biol 25:13–20. https://doi.org/10.1038/s41594-017-0010-8
Bhatt S, Gething PW, Brady OJ et al (2013) The global distribution and burden of dengue. Nature 496:504–507. https://doi.org/10.1038/nature12060
Khetarpal N, Khanna I (2016) Dengue fever : causes, complications, and vaccine strategies. J Immunol Res. https://doi.org/10.1155/2016/6803098
Salles TS, Sá-guimarães TE, Seam E et al (2018) History, epidemiology and diagnostics of dengue in the American and Brazilian contexts : a review. Parasi Vector 11:1–12
Duarte JL, Diaz-quijano FA, Batista AC, Giatti LL (2019) Major article climatic variables associated with dengue incidence in a city of the Western Brazilian Amazon region. J Brazilian Soc Trop Med 52:1–8
Agumadu VC, Ramphul K (2018) Zika Virus: a review of literature. Cureus 10:1–5. https://doi.org/10.7759/cureus.3025
de Araújo TVB, Rodrigues LC, de Alencar Ximenes RA et al (2016) Association between zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet Infect Dis 16:1356–1363. https://doi.org/10.1016/S1473-3099(16)30318-8
De SWV, De AMDFPM, Vazquez E et al (2018) Microcephaly epidemic related to the zika virus and living conditions in recife, Northeast Brazil. BMC Public Health 18:1–7. https://doi.org/10.1186/s12889-018-5039-z
Cauchemez S, Besnard M, Bompard P et al (2016) Association between zika virus and microcephaly in French polynesia, 2013–15: a retrospective study. Lancet 6736:1–8. https://doi.org/10.1016/S0140-6736(16)00651-6
Karkhah A, Nouri HR, Javanian M et al (2018) Zika virus: epidemiology, clinical aspects, diagnosis, and control of infection. Eur J Clin Microbiol Infect Dis. https://doi.org/10.1007/s10096-018-3354-z
Waggoner JJ, Pinsky BA (2016) Zika virus: diagnostics for an emerging pandemic threat. J Clin Microbiol 54:860–867. https://doi.org/10.1128/jcm.00279-16
Dai L, Song J, Lu X et al (2016) Structures of the Zika virus envelope protein and its complex with a flavivirus broadly protective antibody. Cell Host Microbe 19:696–704. https://doi.org/10.1016/j.chom.2016.04.013
Tsai W-Y, Youn HH, Brites C et al (2017) Distinguishing secondary dengue virus infection from zika virus infection with previous dengue by a combination of three serological tests. Clin Infect Dis 65:1829–1836. https://doi.org/10.1093/cid/cix672
Felix AC, Souza NCS, Figueiredo WM et al (2017) Cross reactivity of commercial anti-dengue immunoassays in patients with acute Zika virus infection. J Med Virol 89:1477–1479. https://doi.org/10.1002/jmv.24789
Zhang B, Pinsky BA, Ananta JS et al (2017) Diagnosis of zika virus infection on a nanotechnology platform. Nat Med 23:548–550. https://doi.org/10.1038/nm.4302
Johnson S, Fremont DH, Sukupolvi-Petty S et al (2010) Structure and function analysis of therapeutic monoclonal antibodies against dengue virus type 2. J Virol 84:9227–9239. https://doi.org/10.1128/jvi.01087-10
Shrestha B, Nelson CA, Brien JD et al (2010) The development of therapeutic antibodies that neutralize homologous and heterologous genotypes of dengue virus type 1. PLoS Pathog 6:1–18. https://doi.org/10.1371/journal.ppat.1000823
Zhao H, Fernandez E, Dowd KA et al (2016) Structural Basis of zika virus-specific antibody protection. Cell 166:1016–1027. https://doi.org/10.1016/j.cell.2016.07.020
Rockstroh A, Moges B, Barzon L et al (2017) Specific detection of dengue and zika virus antibodies using envelope proteins with mutations in the conserved fusion loop. Emerg Microbes Infect 6:1–9. https://doi.org/10.1038/emi.2017.87
Premkumar L, Collins M, Graham S et al (2018) Development of envelope protein antigens to serologically differentiate zika virus infection from dengue virus infection. J Clin Microbiol 56:1–13. https://doi.org/10.1128/JCM.01504-17
Maillère B, Boyton RJ, Dorner M et al (2018) T cell immunity to zika virus targets immunodominant epitopes that show cross-reactivity with other flaviviruses. Sci Rep 8:1–12. https://doi.org/10.1038/s41598-017-18781-1
Chen WH, Chou FP, Wang YK et al (2017) Characterization and epitope mapping of dengue virus type 1 specific monoclonal antibodies. Virol J 14:1–11. https://doi.org/10.1186/s12985-017-0856-8
Vasques RM, Lacorte C, da Luz LL et al (2019) Development of a new tobamovirus-based viral vector for protein expression in plants. Mol Biol Rep 46:97–103. https://doi.org/10.1007/s11033-018-4449-4
Tripathi NK, Shrivastva A, Pattnaik P et al (2007) Production, purification and characterization of recombinant dengue multiepitope protein. Biotechnol Appl Biochem 46:105. https://doi.org/10.1042/ba20060090
Maldaner FR, Aragão FJL, Dos Santos FB et al (2013) Dengue virus tetra-epitope peptide expressed in lettuce chloroplasts for potential use in dengue diagnosis. Appl Microbiol Biotechnol 97:5721–5729. https://doi.org/10.1007/s00253-013-4918-6
Chaves LCS, Ribeiro BM, Blissard GW (2018) Production of GP64-free virus-like particles from baculovirus-infected insect cells. J Gen Virol 99:265–274. https://doi.org/10.1099/jgv.0.001002
Liu F, Wu X, Li L et al (2013) Use of baculovirus expression system for generation of virus-like particles: successes and challenges. Protein Expr Purif 90:104–116. https://doi.org/10.1016/j.pep.2013.05.009
Ardisson-Araújo DMP, Rocha JR, Da Costa MHO et al (2013) A baculovirus-mediated strategy for full-length plant virus coat protein expression and purification. Virol J 10:1–9. https://doi.org/10.1186/1743-422X-10-262
Sambrook J, Russel DW (2012) Molecular cloning: a laboratory manual. Cold Spring Harbor, New York p 1890-1890
O’Reilly DR, Miller L, Luckow VA (1992) Baculovirus expression vectors: a laboratory manual. Oxford University Press, New York
AnandaRao R, Swaminathan S, Fernando S et al (2005) A custom-designed recombinant multiepitope protein as a dengue diagnostic reagent. Protein Expr Purif 41:136–147. https://doi.org/10.1016/j.pep.2005.01.009
Rao RA, Swaminathan S, Fernando S et al (2006) Recombinant multiepitope protein for early detection of dengue infections. Clin Vaccine Immunol 13:59–67. https://doi.org/10.1128/CVI.13.1.59-67.2006
Tripathi NK, Babu JP, Shrivastva A et al (2008) Production and characterization of recombinant dengue virus type 4 envelope domain III protein. J Biotechnol 134:278–286. https://doi.org/10.1016/j.jbiotec.2008.02.001
Tripathi NK, Shrivastava A, Rao BKC, PVL, (2011) Recombinant dengue virus type 3 envelope domain III protein from Escherichia coli. Biotechnol J 6:604–608. https://doi.org/10.1002/biot.201000399
Álvarez-Rodríguez LM, Ramos-Ligonio A, Rosales-Encina JL et al (2012) Expression, purification, and evaluation of diagnostic potential and immunogenicity of a recombinant NS3 protein from all serotypes of dengue virus. J Trop Med. https://doi.org/10.1155/2012/956875
Tripathi NK, Shrivastva A, Pattnaik P et al (2007) Production of IgM specific recombinant dengue multiepitope protein for early diagnosis of dengue infection. Biotechnol Prog 23:488–493. https://doi.org/10.1021/bp0602698
Safronetz D, Sloan A, Stein DR et al (2017) Evaluation of 5 commercially available Zika virus immunoassays. Emerg Infect Dis 23:1577–1580. https://doi.org/10.3201/eid2309.162043
Rockstroh A, Barzon L, Pacenti M et al (2015) Recombinant envelope-proteins with mutations in the conserved fusion loop allow specific serological diagnosis of dengue-infections. PLoS Negl Trop Dis 9:1–12. https://doi.org/10.1371/journal.pntd.0004218
Zaidi MB, CedilloBarron L, González Y, Almeida ME et al (2020) Serological tests reveal significant cross-reactive human antibody responses to Zika and Dengue viruses in the Mexican population. Acta Trop. https://doi.org/10.1016/j.actatropica.2019.105201
Acknowledgements
This work was supported by grants from Fundação de Apoio a Pesquisa do Distrito Federal (FAP/DF Grant Numbers 193.001532/2016 and 0193.000416/2016) and was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001". Our thanks to the researchers of the “Zika Plan” group (734584-European Union’s Horizon 2020) and also to Apoio a Pesquisa do Estado de Goiás (FAPEG) (Project Number 201710267001241) for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lopes-Luz, L., Junqueira, I.C., da Silveira, L.A. et al. Dengue and Zika virus multi-epitope antigen expression in insect cells. Mol Biol Rep 47, 7333–7340 (2020). https://doi.org/10.1007/s11033-020-05772-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05772-1