Abstract
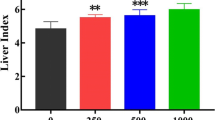
Hormesis is the dose–response pattern of the biological responses to toxic chemicals, characterized by low-dose stimulation and high-dose inhibition. Although it is known that some cell types exhibit an adaptive response to low levels of cytotoxic agents, its molecular mechanism is still unclear and it has yet to be established whether this is a universal phenomenon that occurs in all cell types in response to exposure to every chemical. Trichloroethylene (TCE) is an organic solvent widely used and is released into the atmosphere from industrial degreasing operations. Acute (short-term) and chronic (long-term) inhalation exposure to trichloroethylene can affect the human health. In order to elucidate a cell-survival adaptive response of L-02 liver cells exposed to low dose of TCE, CCK-8 assay was used to assess cytotoxicity, and examined the possible mechanisms of hormesis by proteomics technology. We found that exposure of L-02 liver cells to low level of TCE resulted in adaptation to further exposure to higher level, about 1,000 protein-spots were obtained by two-dimensional electrophoresis (2-DE) and five protein spots were identified by matrix-assisted laser desorption/ionization mass spectrometry and tandem mass spectrometry sequencing of tryptic peptides. Our results suggest that a relationship may exist between identified proteins and TCE-induced hormesis, which are very useful for further study of the mechanism and risk assessment of TCE.







Similar content being viewed by others
References
Calabrese EJ, Baldwin LA (2003) Hormesis: the dose–response revolution. Annu Rev Pharmacol Toxicol 43:175–197. doi:10.1146/annurev.pharmtox.43.100901.140223
Yu-Fei D, Yu-Xin Z (2003) The renew scan of central dogma in toxicology—the dose–effect relationship of intoxicant excitability and the effect to the development of toxicology. Foreign Med Sci 30:246–249 Section of Hygiene
Calabrese EJ (2004) Hormesis—basic, generalizable, central to toxicology and a method to improve the risk-assessment process. Int J Occup Environ Health 10:466–467
Calabrese EJ (2005) Toxicological awakenings: the rebirth of hormesis as a central pillar of toxicology. Toxicol Appl Pharmacol 204:1–8. doi:10.1016/j.taap.2004.11.015
Ming-Xia H (2001) Advancement of trichloroethylene in toxicological research. Foreign Med Sci 28:155–158 Section of Hygiene
Gharahdaghi F, Weinberg CR, Meagher DA et al (1999) Mass spectrometric identification of proteins from silver-stained polyacrylamide gel: a method for the removal of silver ions to enhance sensitivity. Electrophoresis 20:601–605. doi:10.1002/(SICI)1522-2683(19990301)20:3<601::AID-ELPS601>3.0.CO;2-6
Myung K, Braastad C, He DM et al (1998) KARP-1 is induced by DNA damage in a p53- and ataxia telangiectasia mutated-dependent fashion. Proc Natl Acad Sci USA 95:7664–7669. doi:10.1073/pnas.95.13.7664
Myung K, He DM, Lee SE et al (1997) KARP-1: a novel leucine zipper protein expressed from the Ku86 autoantigen locus is implicated in the control of DNA-dependent protein kinase activity. EMBO J 16:3172–3184. doi:10.1093/emboj/16.11.3172
Eunju D, Eiichi T, Yasuyuki I et al (2003) Molecular cloning and characterization of rKAB1, which interacts with KARP-1, localizes in the nucleus and protects cells against oxidative death. Mol Cell Biochem 248:77–83. doi:10.1023/A:1024157515342
Camurri L, Codeluppi S, Pedroni C et al (1983) Chromosomal aberrations and sister-chromatid exchanges in workers exposed to styrene. Mutat Res 119:361–369. doi:10.1016/0165-7992(83)90186-0
Kinoshita A, Wanibuchi H, Wei M et al (2006) Hormesis in carcinogenicity of non-genotoxic carcinogens. J Toxicol Pathol 19:111–122. doi:10.1293/tox.19.111
Kuwabara H, Yoneda M, Nagai M et al (2004) A new polyclonal antibody that recognizes a human receptor for hyaluronan mediated motility. Cancer Lett 210:73–80. doi:10.1016/j.canlet.2004.01.004
Rein DT, Roehrig K, Schondorf T et al (2003) Expression of the hyaluronan receptor RHAMM in endometrial carcinomas suggests a role in tumour progression and metastasis. J Cancer Res Clin Oncol 129:161–164
Tolg C, Poon R, Fodde R et al (2003) Genetic deletion of receptor for hyaluronan-mediated motility (RHAMM) attenuates the formation of aggressive fibromatosis (desmoid tumor). Oncogene 22:6873–6882. doi:10.1038/sj.onc.1206811
Maxwell CA, Keats JJ, Belch AR et al (2005) Receptor for hyaluronan-mediated motility correlates with centrosome abnormalities in multiple myeloma and maintains mitotic integrity. Cancer Res 65:850–860
Adele K, Elizabeth MP, James MP (2002) Effects of chlorinated aliphatic hydrocarbons on the fidelity of cell division in human CYP2E1 expression cells. Exp Mol Med 34:83–89
Board PG, Anders MW (2007) Glutathione transferase omega 1 catalyzes the reduction of S-(Phenacyl) glutathiones to acetophenones. Chem Res Toxicol 20:149–154. doi:10.1021/tx600305y
Nebert DW, Vasiliou V (2004) Analysis of the glutathione S-transferase (GST) gene family. Hum Genomics 1:460–464
Townsend DM, Tew KD (2003) The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene 22:7369–7375. doi:10.1038/sj.onc.1206940
Meyer-Hoffert U, Wingertszahn J, Wiedow O (2004) Human leukocyte elastase induces keratinocyte proliferation by epidermal growth factor receptor activation. J Invest Dermatol 123:338–345. doi:10.1111/j.0022-202X.2004.23202.x
Acknowledgments
This study was supported by National Basic Research Program of China (973 Program, No.: 2002CB512903), National Natural Science Foundation of China (30571557), Guangdong Natural Science Foundation (5009153).
Author information
Authors and Affiliations
Corresponding author
Additional information
Hai-Yan Huang and Jian-Jun Liu have contributed equally.
Rights and permissions
About this article
Cite this article
Huang, HY., Liu, JJ., Xi, RR. et al. An investigation of hormesis of trichloroethylene in L-02 liver cells by differential proteomic analysis. Mol Biol Rep 36, 2119–2129 (2009). https://doi.org/10.1007/s11033-008-9424-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-008-9424-z