Abstract
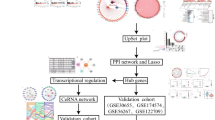
Cerebral ischemia is one of the major causes of death and disability worldwide. Currently, existing approved therapies are based on reperfusion and there is an unmet need to search for drugs with neuroprotective effects. The present study aims to investigate the neuroprotective mechanisms of nitroxoline, a nitro derivative of 8-Hydroxyquinoline, against cerebral ischemia using integrated network pharmacology and molecular docking approaches. Critical analytical tools used were SwissTarget, PharmMapper, BindingDB, DisGeNet, Cytoscape, GeneMANIA, ShinyGo, Metascape, GeneCodis, and Schrodinger GLIDE. Thirty-six overlapping drug and disease targets were identified and used for further analysis. Gene Ontology results showed that nitroxoline enriched the genes involved in biological processes of oxidative stress and apoptotic cell death that are highly implicated in hypoxic injury. KEGG enrichment analysis showed nitroxoline influenced a total of 159 biological pathways, out of which, top pathways involved in cerebral ischemia included longevity regulating pathway, VEGF signaling, EGFR tyrosine kinase inhibitor resistance, IL-17 and HIF-1 pathways, FoxO signaling, and AGE-RAGE pathway. Protein–protein interaction analysis using string database showed PARP1, EGFR, PTEN, BRD4, RAC1, NOS2, MTOR, MAPK3, BCL2, MAPK1, APP, METAP2, MAPK14, SIRT1, PRKAA1, and MCL1 as highly interactive proteins involved in pathogenesis of ischemic stroke regulated by nitroxoline. The highly interactive protein targets were validated by molecular docking studies and molecular dynamic simulations. Amongst all these targets, nitroxoline showed the highest binding affinity towards BRD4 followed by PARP1 and PTEN. Nitroxoline, through network pharmacology analysis, showed a role in regulating proteins, biological processes, and pathways crucial in cerebral ischemia. The current study thus provides a preliminary insight that nitroxoline might be used as a neuroprotectant against cerebral ischemia via modulating the epigenetic reader BRD4 and transcription factors such as RELA, NF-κβ1, and SP1. However, further in-vitro and preclinical studies need to be performed for concrete evidence.















Similar content being viewed by others
Availability of data and materials
Data will be made available on request.
References
Tsao CW, Aday AW, Almarzooq ZI, Anderson CA, Arora P, Avery CL et al (2023) Heart disease and stroke statistics—2023 update: a report from the American Heart Association. Circ 147:e93–e621. https://doi.org/10.1161/CIR.0000000000001123
Nogles TE, Galuska MA (2021) Middle cerebral artery stroke. StatPearls [Internet]: StatPearls Publishing
Ma H, Campbell BC, Parsons MW, Churilov L, Levi CR, Hsu C et al (2019) Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med 380:1795–1803. https://doi.org/10.1056/NEJMoa1813046
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL (2019) Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 50:e344–e418. https://doi.org/10.1161/STR.0000000000000211
Chrostek MR, Fellows EG, Crane AT, Grande AW, Low WC (2019) Efficacy of stem cell-based therapies for stroke. Brain res 1722:146362. https://doi.org/10.1016/j.brainres.2019.146362
Albers GW, Goldstein LB, Hess DC, Wechsler LR, Furie KL, Gorelick PB, Patty H, David SL, Raul GN, Jeffrey LS (2011) Stroke Treatment Academic Industry Roundtable (STAIR) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra-arterial and neuroprotective therapies. Stroke 42:2645–2650. https://doi.org/10.1161/STROKEAHA.111.618850
Yang JL, Yang YR, Chen SD (2019) The potential of drug repurposing combined with reperfusion therapy in cerebral ischemic stroke: a supplementary strategy to endovascular thrombectomy. Life Sci 236:116889. https://doi.org/10.1016/j.lfs.2019.116889
Mrhar A, Kopitar Z, Kozjek F, Presl V, Karba R (1979) Clinical pharmacokinetics of nitroxoline. Int J Clin Pharmacol Biopharm 17:476–481
Bissani Gasparin C, Pilger DA (2023) 8-Hydroxyquinoline, derivatives and metal-complexes: a review of antileukemia activities. ChemistrySelect 8:e202204219. https://doi.org/10.1002/slct.202204219
Mirković B, Renko M, Turk S, Sosič I, Jevnikar Z, Obermajer N et al (2011) Novel mechanism of cathepsin B inhibition by antibiotic nitroxoline and related compounds. ChemMedChem 6:1351–1356. https://doi.org/10.1002/cmdc.201100098
Sun C, Cao N, Wang Q, Liu N, Yang T, Li S, Pan L, Yao J, Zhang Li, Liu M, Zhang G, Xiao X, Liu C (2023) Icaritin induces resolution of inflammation by targeting cathepsin B to prevents mice from ischemia-reperfusion injury. Int Immunopharmacol 116:109850. https://doi.org/10.1016/j.intimp.2023.109850
Sosič I, Mitrović A, Ćurić H, Knez D, Žugelj HB, Štefane B, Kos J, Gobec S (2018) Cathepsin B inhibitors: Further exploration of the nitroxoline core. Bioorganic Med Chem Lett 28:1239–1247. https://doi.org/10.1016/j.bmcl.2018.02.042
Endres M, Meisel A, Biniszkiewicz D, Namura S, Prass K, Ruscher K, Lipski A, Jaenisch R, Moskowitz MA, Dirnagl U (2000) DNA methyltransferase contributes to delayed ischemic brain injury. J Neurosci 20:3175–3181. https://doi.org/10.1523/JNEUROSCI.20-09-03175.2000
Kim HJ, Rowe M, Ren M, Hong JS, Chen PS, Chuang DM (2007) Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J Pharmacol Exp Ther 321:892–901. https://doi.org/10.1124/jpet.107.120188
Zhou Y, Song T, Peng J, Zhou Z, Wei H, Zhou R, Jiang S, Peng J (2016) SIRT1 suppresses adipogenesis by activating Wnt/β-catenin signaling in vivo and in vitro. Oncotarget 7(47):77707–77720. https://doi.org/10.18632/oncotarget.12774
Erkasap S, Erkasap N, Bradford B, Mamedova L, Uysal O, Ozkurt M, Ozyurt R, Kutlay O, Bayram B (2017) The effect of leptin and resveratrol on JAK/STAT pathways and Sirt-1 gene expression in the renal tissue of ischemia/reperfusion induced rats. Bratisl Lek Listy 118(8):443–448. https://doi.org/10.4149/BLL_2017_086
Bai X, He T, Liu Y, Zhang J, Li X, Shi J, Wang K, Han F, Zhang W, Zhang Y, Cai W, Hu D (2018) Acetylation-dependent regulation of notch signaling in macrophages by SIRT1 affects sepsis development. Front Immunol 9:344581. https://doi.org/10.3389/fimmu.2018.00762
Liu L, Zhu X, Zhao T, Yu Y, Xue Y, Zou H (2019) Sirt1 ameliorates monosodium urate crystal–induced inflammation by altering macrophage polarization via the PI3K/Akt/STAT6 pathway. Rheumatology (Oxford) 58(9):1674–1683. https://doi.org/10.1093/rheumatology/kez165
Ma J, Fan H, Cai H, Hu Z, Zhou X, Li F, Chen H, Shen J, Qi S (2021) Promotion of Momordica Charantia polysaccharides on neural stem cell proliferation by increasing SIRT1 activity after cerebral ischemia/reperfusion in rats. Brain Res Bull 170:254–263. https://doi.org/10.1016/j.brainresbull.2021.02.016
Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR (2004) Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 303(5660):1010–1014. https://doi.org/10.1126/science.1092734
Liu Y, Fu N, Su J, Wang X, Li X (2019) Rapid enkephalin delivery using exosomes to promote neurons recovery in ischemic stroke by inhibiting neuronal p53/caspase-3. Biomed Res Int 2019:4273290. https://doi.org/10.1155/2019/4273290
Hu W, Chan CS, Wu R, Zhang C, Sun Y, Song JS, Tang LH, Levine AJ, Feng Z (2010) Negative regulation of tumor suppressor p53 by microRNA miR-504. Mol Cell 38:689–699. https://doi.org/10.1016/j.molcel.2010.05.027
Teertam SK, Jha S, Prakash Babu P (2020) Up-regulation of Sirt1/miR-149-5p signaling may play a role in resveratrol induced protection against ischemia via p53 in rat brain. J Clin Neurosci 72:402–411. https://doi.org/10.1016/j.jocn.2019.11.043
Jover-Mengual T, Hwang JY, Byun HR, Court-Vazquez BL, Centeno JM, Burguete MC, Zukin RS (2021) The role of NF-kB triggered inflammation in cerebral ischemia. Front Cell Neurosci 15(633610):1–8. https://doi.org/10.3389/fncel.2021.633610
Diamant G, Dikstein R (2013) Transcriptional control by NF-κB: elongation in focus. Biochim Biophys Acta 1829:937–945. https://doi.org/10.1016/j.bbagrm.2013.04.007
Van Hau T, Ruankham W, Suwanjang W, Songtawee N, Wongchitrat P, Pingaew R, Prachayasittikul V, Prachayasittikul S, Phopin K (2019) Repurposing of Nitroxoline drug for the prevention of neurodegeneration. Chem Res Toxicol 32(11):2182–2191. https://doi.org/10.1021/acs.chemrestox.9b00183
Araújo IM, Verdasca MJ, Leal EC, Bahr BA, Ambrósio AF, Carvalho AP, Carvalho CM (2004) Early calpain-mediated proteolysis following AMPA receptor activation compromises neuronal survival in cultured hippocampal neurons. J Neurochem 91:1322–1331. https://doi.org/10.1111/j.1471-4159.2004.02811.x
Das A, Banik NL, Ray SK (2006) Mechanism of apoptosis with the involvement of calpain and caspase cascades in human malignant neuroblastoma SH-SY5Y cells exposed to flavonoids. Int J Cancer 119(11):2575–2585. https://doi.org/10.1002/ijc.22228
Gay NH, Phopin K, Suwanjang W, Songtawee N, Ruankham W, Wongchitrat P, Prachayasittikul S, Prachayasittikul V (2018) Neuroprotective effects of phenolic and carboxylic acids on oxidative stress-induced toxicity in human neuroblastoma SH-SY5Y cells. Neurochem Res 43:619–636. https://doi.org/10.1007/s11064-017-2463-x
Xiong DD, Qin Y, Xu WQ, He RQ, Wu HY, Wei DM, Zeng JJ, Dang YW, Chen G (2018) A network pharmacology-based analysis of multi-target, multi-pathway, multi-compound treatment for ovarian serous cystadenocarcinoma. Clin Drug Investig 38(10):909–925. https://doi.org/10.1007/s40261-018-0683-8
Hopkins AL (2008) Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol 4:682–690. https://doi.org/10.1038/nchembio.118
Qi Q, Li R, Li HY, Cao YB, Bai M, Fan XJ, Wang SY, Zhang B, Li S (2016) Identification of the anti-tumor activity and mechanisms of nuciferine through a network pharmacology approach. Acta Pharmacol Sin 37(7):963–972. https://doi.org/10.1038/aps.2016.53
Chen H, He Y, Chen S, Qi S, Shen J (2020) Therapeutic targets of oxidative/nitrosative stress and neuroinflammation in ischemic stroke: applications for natural product efficacy with omics and systemic biology. Pharmacol Res 158:104877. https://doi.org/10.1016/j.phrs.2020.104877
Daina A, Michielin O, Zoete V (2019) SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res 47(W1):W357–W364. https://doi.org/10.1093/nar/gkz382
Chen X, Lin Y, Liu M, Gilson MK (2002) The binding database: data management and interface design. Bioinformatics 18(1):130–139. https://doi.org/10.1093/bioinformatics/18.1.130
Liu X, Ouyang S, Yu B, Liu Y, Huang K, Gong J, Zheng S, Li Z, Li H, Jiang H (2010) PharmMapper server: a web server for potential drug target identification using pharmacophore mapping approach. Nucleic Acids Res 38:W609–W614. https://doi.org/10.1093/nar/gkq300
Piñero J, Ramírez-Anguita JM, Saüch-Pitarch J, Ronzano F, Centeno E, Sanz F, Furlong LI (2020) The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res 48(D1):D845–D855. https://doi.org/10.1093/nar/gkz1021
Ng YS, Stein J, Ning M, Black-Schaffer RM (2007) Comparison of clinical characteristics and functional outcomes of ischemic stroke in different vascular territories. Stroke 38(8):2309–2314. https://doi.org/10.1161/STROKEAHA.106.475483
Fluri F, Schuhmann MK, Kleinschnitz C (2015) Animal models of ischemic stroke and their application in clinical research. Drug Des Devel Ther 9:3445–3454. https://doi.org/10.2147/DDDT.S56071
Heberle H, Meirelles GV, da Silva FR, Telles GP, Minghim R (2015) InteractiVenn: a web-based tool for the analysis of sets through Venn diagrams. BMC Bioinformatics 16(1):1–7. https://doi.org/10.1186/s12859-015-0611-3
Mostafavi S, Ray D, Warde-Farley D, Grouios C, Morris Q (2008) GeneMANIA: a real-time multiple association network integration algorithm for predicting gene function. Genome Biol 9(S4):1–15. https://doi.org/10.1186/gb-2008-9-s1-s4
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G (2000) Gene ontology: tool for the unification of biology. Nat Genet 25(1):25–29. https://doi.org/10.1038/75556
Ge SX, Jung D, Yao R (2020) ShinyGO: a graphical gene-set enrichment tool for animals and plants. Bioinformatics 36(8):2628–2629. https://doi.org/10.1093/bioinformatics/btz931
Garcia-Moreno A, López-Domínguez R, Villatoro-García JA, Ramirez-Mena A, Aparicio-Puerta E, Hackenberg M, Pascual-Montano A, Carmona-Saez P (2022) Functional enrichment analysis of regulatory elements. Biomedicines 10(3):590. https://doi.org/10.3390/biomedicines10030590
Mi H, Thomas P (2009) PANTHER pathway: an ontology-based pathway database coupled with data analysis tools. Protein Networks and Pathway Analysis 563:123–140. https://doi.org/10.1007/978-1-60761-175-2_7
Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT (2006) Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein− ligand complexes. J Med Chem 49(21):6177–6196. https://doi.org/10.1021/jm051256o
Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, Banks JL (2004) Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem 47(7):1750–1759. https://doi.org/10.1021/jm030644s
Ahmad I, Kumar D, Patel H (2022) Computational investigation of phytochemicals from Withania somnifera (Indian ginseng/ashwagandha) as plausible inhibitors of GluN2B-containing NMDA receptors. J Biomol Struct Dyn 40(17):7991–8003. https://doi.org/10.1080/07391102.2021.1905553
Rao NL, Kotian GB, Shetty JK, Shelley BP, Dmello MK, Lobo EC, Shankar SP, Almeida SD, Shah SR (2022) Receptor for advanced glycation end product, organ crosstalk, and pathomechanism targets for comprehensive molecular therapeutics in diabetic ischemic stroke. Biomolecules 12(11):1712. https://doi.org/10.3390/biom12111712
Pawluk H, Woźniak A, Grześk G, Kołodziejska R, Kozakiewicz M, Kopkowska E, Grzechowiak E, Kozera G (2020) The role of selected pro-inflammatory cytokines in pathogenesis of ischemic stroke. Clin Interv Aging 15:469–484. https://doi.org/10.2147/CIA.S233909
Uzdensky AB (2019) Apoptosis regulation in the penumbra after ischemic stroke: expression of pro-and antiapoptotic proteins. Apoptosis 24:687–702. https://doi.org/10.1007/s10495-019-01556-6
Jiang Q, Geng X, Warren J, Eugene Paul Cosky E, Kaura S, Stone C, Li F, Ding Y (2020) Hypoxia inducible factor-1α (HIF-1α) mediates NLRP3 inflammasome-dependent-pyroptotic and apoptotic cell death following ischemic stroke. Neuroscience 448:126–139. https://doi.org/10.1016/j.neuroscience.2020.09.036
McCullough LD, Tarabishy S, Liu L, Benashski S, Xu Y, Ribar T, Means A, Li J (2013) Inhibition of calcium/calmodulin-dependent protein kinase kinase β and calcium/calmodulin-dependent protein kinase IV is detrimental in cerebral ischemia. Stroke 44(9):2559–2566. https://doi.org/10.1161/STROKEAHA.113.001030
Huang HY, Lin YC, Cui S, Huang Y, Tang Y, Xu J, Bao J, Li Y, Wen J, Zuo H, Wang W, Li J, Ni J, Ruan Y, Li L, Chen Y, Xie Y, Zhu Z, Cai X, Chen X, Yao L, Chen Y, Luo Y, LuXu S, Luo M, Chiu CM, Ma K, Zhu L, Cheng GJ, Bai C, Chiang YC, Wang L, Wei F, Lee TY, Huang HD (2022) miRTarBase update 2022: an informative resource for experimentally validated miRNA–target interactions. Nucleic Acids Res 50(D1):D222–D230. https://doi.org/10.1093/nar/gkab1079
Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, Philpott M, Munro S, McKeown MR, Wang Y, Christie AL, West N, Cameron MJ, Schwartz B, Heightman TD, La Thangue N, French CA, Wiest O, Kung AL, Knapp S, Bradner JE (2010) Selective inhibition of BET bromodomains. Nature 468:1067–1073. https://doi.org/10.1038/nature09504
Dawicki-McKenna JM, Langelier MF, DeNizio JE, Riccio AA, Cao CD, Karch KR, McCauley M, Steffen JD, Black BE, Pascal JM (2015) PARP-1 activation requires local unfolding of an autoinhibitory domain. Mol Cell 60(5):755–768. https://doi.org/10.1016/j.molcel.2015.10.013
Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y, Dixon JE, Pandolfi P, Pavletich NP (1999) Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell 99:323–334. https://doi.org/10.1016/s0092-8674(00)81663-3
Cao D, Wang M, Qiu X, Liu D, Jiang H, Yang N, Xu RM (2015) Structural basis for allosteric, substrate-dependent stimulation of SIRT1 activity by resveratrol. Genes Dev 29(12):1316–1325
Gill AL, Frederickson M, Cleasby A, Woodhead SJ, Carr MG, Woodhead AJ, Walker MT, Congreve MS, Devine LA, Tisi D, O’Reilly M, Seavers LC, Davis DJ, Curry J, Anthony R, Padova A, Murray CW, Carr RA, Jhoti H (2005) Identification of novel p38α MAP kinase inhibitors using fragment-based lead generation. J Med Chem 48(2):414–426. https://doi.org/10.1021/jm049575n
Aronov AM, Baker C, Bemis GW, Cao J, Chen G, Ford PJ, Germann UA, Green J, Hale MR, Jacobs M, Janetka JW, Maltais F, Martinez-Botella G, Namchuk MN, Straub J, Tang Q, Xie X (2007) Flipped out: structure-guided design of selective pyrazolylpyrrole ERK inhibitors. J Med Chem 50:1280–1287. https://doi.org/10.1021/jm061381f
Chaikuad A, Tacconi EM, Zimmer J, Liang Y, Gray NS, Tarsounas M, Knapp S (2014) A unique inhibitor binding site in ERK1/2 is associated with slow binding kinetics. Nat Chem Biol 10(10):853–860. https://doi.org/10.1038/nchembio.1629
Choi J, Chen J, Schreiber SL, Clardy J (1996) Structure of the FKBP12-rapamycin complex interacting with binding domain of human FRAP. Science 273(5272):239–242. https://doi.org/10.1126/science.273.5272.239
Li X, Wang L, Zhou XE, Ke J, de Waal PW, Gu X, Tan MH, Wang D, Wu D, Xu HE, Melcher K (2015) Structural basis of AMPK regulation by adenine nucleotides and glycogen. Cell Res 25(1):50–66. https://doi.org/10.1038/cr.2014.150
Garcin ED, Arvai AS, Rosenfeld RJ, Kroeger MD, Crane BR, Andersson G, Andrews G, Hamley PJ, Mallinder PR, Nicholls DJ, St-Gallay SA, Tinker AC, Gensmantel NP, Mete A, Cheshire DR, Connolly S, Stuehr DJ, Aberg A, Wallace AV, Tainer JA, Getzoff ED (2008) Anchored plasticity opens doors for selective inhibitor design in nitric oxide synthase. Nat Chem Biol 4(11):700–707. https://doi.org/10.1038/nchembio.115
Stamos J, Sliwkowski MX, Eigenbrot C (2002) Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J Biol Chem 277(48):46265–46272. https://doi.org/10.1074/jbc.M207135200
Casara P, Davidson J, Claperon A, Le Toumelin-Braizat G, Vogler M, Bruno A, Chanrion M, Lysiak-Auvity G, Le Diguarher T, Starck JB, Chen I, Whitehead N, Graham C, Matassova N, Dokurno P, Pedder C, Wang Y, Qiu S, Girard AM, Schneider E, Gravé F, Studeny A, Guasconi G, Rocchetti F, Maïga S, Henlin JM, Colland F, Kraus-Berthier L, Le Gouill S, Dyer MJS, Hubbard R, Wood M, Amiot M, Cohen GM, Hickman JA, Morris E, Murray J, Geneste O (2018) S55746 is a novel orally active BCL-2 selective and potent inhibitor that impairs hematological tumor growth. Oncotarget 9(28):20075–20088. https://doi.org/10.18632/oncotarget.24744
Krauthammer M, Kong Y, Ha BH, Evans P, Bacchiocchi A, McCusker JP, Cheng E, Davis MJ, Goh G, Choi M, Ariyan S, Narayan D, Dutton-Regester K, Capatana A, Holman EC, Bosenberg M, Sznol M, Kluger HM, Brash DE, Stern DF, Materin MA, Lo RS, Mane S, Ma S, Kidd KK, Hayward NK, Lifton RP, Schlessinger J, Boggon TJ, Halaban R (2012) Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet 44(9):1006–1014. https://doi.org/10.1038/ng.2359
Sheppard GS, Wang J, Kawai M, BaMaung NY, Craig RA, Erickson SA, Lynch L, Patel J, Yang F, Searle XB, Lou P, Park C, Kim KH, Henkin J, Lesniewski R (2004) 3-Amino-2-hydroxyamides and related compounds as inhibitors of methionine aminopeptidase-2. Bioorg Med Chem Lett 14(4):865–868. https://doi.org/10.1016/j.bmcl.2003.12.031
Utta S, Gullá S, Chen TS, Fire E, Grant RA, Keating AE (2010) Determinants of BH3 binding specificity for Mcl-1 versus Bcl-xL. J Mol Biol 398(5):747–762. https://doi.org/10.1016/j.jmb.2010.03.058
Crescenzi O, Tomaselli S, Guerrini R, Salvadori S, D’Ursi AM, Temussi PA, Picone D (2002) Solution structure of the Alzheimer amyloid β-peptide (1–42) in an apolar microenvironment: Similarity with a virus fusion domain. Eur J Biochem 269(22):5642–5648. https://doi.org/10.1046/j.1432-1033.2002.03271.x
Nogales C, Mamdouh ZM, List M, Kiel C, Casas AI, Schmidt HHHW (2022) Network pharmacology: curing causal mechanisms instead of treating symptoms. Trends Pharmacol Sci 43:136–150. https://doi.org/10.1016/j.tips.2021.11.004
Naber KG, Niggemann H, Stein G, Stein G (2014) Review of the literature and individual patients’ data meta-analysis on efficacy and tolerance of nitroxoline in the treatment of uncomplicated urinary tract infections. BMC Infect Dis 14:1–16. https://doi.org/10.1186/s12879-014-0628-7
Clarkson AN, Huang BS, MacIsaac SE, Mody I, Carmichael ST (2010) Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature 468(7321):305–309. https://doi.org/10.1038/nature09511
Fraser MM, Zhu X, Kwon CH, Uhlmann EJ, Gutmann DH, Baker SJ (2004) Pten loss causes hypertrophy and increased proliferation of astrocytes in vivo. Cancer Res 64:7773–7779. https://doi.org/10.1158/0008-5472.CAN-04-2487
Li W, Huang R, Shetty RA, Thangthaeng N, Liu R, Chen Z, Sumien N, Rutledge M, Dillon GH, Yuan F, Forster MJ, Simpkins JW, Yang SH (2014) Transient focal cerebral ischemia induces long-term cognitive function deficit in an experimental ischemic stroke model. Neurobiol Dis 59:18–25. https://doi.org/10.1016/j.nbd.2013.06.014
Li W, Huang R, Chen Z, Yan LJ, Simpkins JW, Yang SH (2014) PTEN degradation after ischemic stroke: a double-edged sword. Neuroscience 274:153–161. https://doi.org/10.1016/j.neuroscience.2014.05.027
Liu S, Luo W, Wang Y (2022) Emerging role of PARP-1 and PARthanatos in ischemic stroke. J Neurochem 160:74–87. https://doi.org/10.1111/jnc.15464
Jackson CW, Xu J, Escobar I, Saul I, Fagerli E, Dave KR, Perez-Pinzon MA (2023) Resveratrol preconditioning downregulates PARP1 protein to alleviate PARP1-mediated cell death following cerebral ischemia. Transl Stroke Res. https://doi.org/10.1007/s12975-022-01119-z
Li X, Zhu H, Wen J, Huang J, Chen Y, Tian M, Ren J, Zhou L, Yang Q (2022) Inhibition of BRD4 decreases fibrous scarring after ischemic stroke in rats by inhibiting the phosphorylation of Smad2/3. Brain Res 1797:148126. https://doi.org/10.1016/j.brainres.2022.148126
Zhou Y, Gu Y, Liu J (2019) BRD4 suppression alleviates cerebral ischemia-induced brain injury by blocking glial activation via the inhibition of inflammatory response and pyroptosis. Biochem Biophys Res Commun 519(3):481–488. https://doi.org/10.1016/j.bbrc.2019.07.097
Liu L, Yang C, Lavayen BP, Tishko RJ, Larochelle J, Candelario-Jalil E (2022) Targeted BRD4 protein degradation by dBET1 ameliorates acute ischemic brain injury and improves functional outcomes associated with reduced neuroinflammation and oxidative stress and preservation of blood–brain barrier integrity. J Neuroinflammation 19(1):168. https://doi.org/10.1186/s12974-022-02533-8
Pan Z, Ma G, Kong L, Du G (2021) Hypoxia-inducible factor-1: Regulatory mechanisms and drug development in stroke. Pharmacol Res 170:105742. https://doi.org/10.1016/j.phrs.2021.105742
Marques-Oliveira R, Alfenim AR, Soares P, Borges F, Remião F, Fernandes C, Silva R (2023) New 8-hydroxyquinoline derivatives as promising therapeutic approaches targeting neurodegeneration. Scient Lett https://doi.org/10.48797/sl.2023.92
Zhao Y, Xie Z, Lin J, Liu P (2017) MiR-144-3p inhibits cell proliferation and induces apoptosis in multiple myeloma by targeting c-Met. Am J Transl Res 9(5):2437–2446
Li Y, Zhao Y, Cheng M, Qiao Y, Wang Y, Xiong W, Yue W (2018) Suppression of microRNA-144-3p attenuates oxygen–glucose deprivation/reoxygenation-induced neuronal injury by promoting Brg1/Nrf2/ARE signaling. J Biochem Mol Toxicol 32(4):e22044. https://doi.org/10.1002/jbt.22044
Ren W, Zhao F, Han Y, Liu Z, Zhai J, Jia K (2022) Muscone improves hypoxia/reoxygenation (H/R)-induced neuronal injury by blocking HMGB1/TLR4/NF-κB pathway via modulating microRNA-142. PeerJ 10:e13523. https://doi.org/10.7717/peerj.13523
Wang N, Zhang L, Lu Y, Zhang M, Zhang Z, Wang K, Lv J (2017) Down-regulation of microRNA-142-5p attenuates oxygen-glucose deprivation and reoxygenation-induced neuron injury through up-regulating Nrf2/ARE signaling pathway. Biomed Pharmacother 89:1187–1195. https://doi.org/10.1016/j.biopha.2017.03.011
Schwalm MP, Knapp S (2022) BET bromodomain inhibitors. Curr Opin Chem Biol 68:102148. https://doi.org/10.1016/j.cbpa.2022.102148
Wang N, Wu R, Tang D, Kang R (2021) The BET family in immunity and disease. Signal Transduct Target Ther 6(1):23. https://doi.org/10.1038/s41392-020-00384-4
Funding
The authors would like to thank the ‘Department of science and technology, Fund for improvement of S&T infrastructure’ (DST-FIST), Government of India for the grant provided (Grant No. SR/FST/College-054/2017) to strengthen the instrumentation facility.
Author information
Authors and Affiliations
Contributions
NV: Conceptualization, network pharmacology studies, data analysis and interpretation., writing of the manuscript; MB: Molecular Docking studies; data analysis and interpretation. LKB: Conceptualization, study design, data analysis and interpretation, reviewing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vadak, N., Borkar, M.R. & Bhatt, L.K. Deciphering neuroprotective mechanism of nitroxoline in cerebral ischemia: network pharmacology and molecular modeling-based investigations. Mol Divers (2024). https://doi.org/10.1007/s11030-023-10791-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11030-023-10791-8