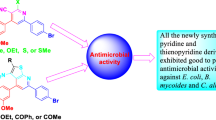
Abstract
The synthesis of 5H-imidazo[4,5-c]pyridines analogues (1a − 1h) and 4H-imidazo[4,5-b]pyridines (3a − 3c) was achieved by reacting 3,4-diaminopyridine or 2,3-diaminopyridine with Na2S2O5 adduct of corresponding benzaldehydes (a1 - a8). Alkylation of compounds (1a − 1h) and (3a − 3c) using 4-chlorobenzyl and /or butyl bromide under basic conditions (K2CO3, DMF) predominantly resulted in the formation of N5 regioisomers (2a − 2l) and N4,3 regioisomers (4a − 4c1,2), respectively. The N5,4,3-regioisomeric structures were confirmed using 2D-NOESY (Nuclear Overhauser Effect Spectroscopy) and HMBC (Heteronuclear Multiple Bond Correlation) spectra. The antibacterial and antifungal activities of the synthesized compounds (2a − 2g, 4a − 5d) were evaluated in vitro against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Methicillin resistant S. aureus, Enterococcus faecalis and Candida albicans, Candida parapsilosis. Among the synthesized compounds, promising activities were observed with compounds 2g, 2h, 4a and 4b with lowest MIC values (4–8 µg/mL). The compounds 2i, 2j, 2k, 2l showed moderate activity. Additionally, a computational approach (ADMETlab 2.0) was used to evaluate the drug likeness properties of the compounds.






Similar content being viewed by others

References
Dyminska L (2015) Imidazopyridines as a source of biological activity and their pharmacological potentials-infrared and raman spectroscopic evidence of their content in pharmaceuticals and plant materials. Bioorg Med Chem 23:6087–6099. https://doi.org/10.1016/j.bmc.2015.07.045
Krause M, Foks H, Gobis K (2017) Pharmacological potential and synthetic approaches of imidazo[4,5-b]pyridine and imidazo[4,5-c]pyridine derivatives. Molecules 22:399. https://doi.org/10.3390/molecules22030399
Cheng CC, Shipps GW Jr, Yang Z, Sun B, Kawahata N, Soucy KA, Soriano A, Orth P, Xiao L, Mann P, Black T (2009) Discovery and optimization of antibacterial AccC inhibitors. Bioorg Med Chem Lett 19:6507–6514. https://doi.org/10.1016/j.bmcl.2009.10.057
Jose G, Kumara THS, Nagendrappa G, Sowmya HBV, Jasinski JP, Millikan SP, Chandrika N, More SS, Harish BG (2014) New polyfunctional imidazo[4,5-c]pyridine motifs: synthesis, crystal studies, docking studies and antimicrobial evaluation. Eur J Med Chem 77:288–297. https://doi.org/10.1016/j.ejmech.2014.03.019
Aridoss G, Balasubramanian S, Parthiban P, Kabilan S (2006) Synthesis and in vitro microbiological evaluation of imidazo(4,5-b)pyridinylethoxypiperidones. Eur J Med Chem 41:268–275. https://doi.org/10.1016/j.ejmech.2005.10.014
Wu D, Liu M, Li Z, Dang M, Liu X, Li J, Huang L, Ren Y, Zhang Z, Liu W, Liu A (2019) Synthesis and fungicidal activity of novel imidazo[4,5-b]pyridine derivatives. Heterocycl Commun 25:8–14. https://doi.org/10.1515/hc-2019-0003
Khoje AD, Charnock C, Wan B, Franzblau S, Gundersen LL (2011) Synthesis and antimycobacterial activities of non-purine analogs of 6-aryl-9-benzylpurines: Imidazopyridines, pyrrolopyridines, benzimidazoles, and indoles. Bioorg Med Chem 19:3483–3491. https://doi.org/10.1016/j.bmc.2011.04.023
Huang W, Zhang Z, Barros-Alvarez X, Koh CY, Ranade RM, Gillespie JR, Creason SA, Shibata S, Verlinde CLMJ, Hol WGJ, Buckner FS, Fan E (2016) Structure-guided design of novel Trypanosoma brucei Methionyl-tRNA synthetase inhibitors. Eur J Med Chem 124:1081–1092. https://doi.org/10.1016/j.ejmech.2016.10.024
Puskullu MO, Doganc F, Ozden S, Sahin E, Celik I, Goker H (2021) Synthesis, NMR, X-ray crystallography and DFT studies of some regioisomers possessing imidazole heterocycles. J Mol Struct 1243:130811. https://doi.org/10.1016/j.molstruc.2021.130811
Doganc F, Alp M, Karabay A, Koç A, Eren G, Göker H (2021) Synthesis and cytotoxicity of come Imidazo[4,5-b]pyridine derivatives and their regioselective N-alkylation. ChemistrySelect 6:1519–1525. https://doi.org/10.1002/slct.202004584
Karaaslan C, Doganc F, Alp M, Koc A, Karabay AZ, Göker H (2020) Regioselective N-alkylation of some imidazole-containing heterocycles and their in vitro anticancer evaluation. J Mol Struct 1205:127673. https://doi.org/10.1016/j.molstruc.2019.127673
Middleton RW, Wibberley DG (1980) Synthesis of imidazo[4,5-b] and [4,5-c]pyridines. J Heterocycl Chem 17:1757. https://doi.org/10.1002/jhet.5570170824
Kim JD, Yun HC, Kim SY, Kim IW, Kim JY, Kim KP, Song YJ, Choi HJ, Shim WJ, Shin KS, Hyun JH, Lee BY (2006) Novel benzimidazol derivatives and pharmaceutical composition comprising the same. WO 2006/080821 A1
Huang HC, Chamberlain TS, Settle SL, Joy WD, Siegel NR, Bell LD (2000) Bicyclic imidazolyl derivatives as phosphodiesterase inhibitors, pharmaceutical compositions and method of use. US 6,130,333
ISO 20776-1 (2006) Clinical laboratory testing and in vitro diagnostic test systems-susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility test devices-part 1: reference method for testing the in vitro activity of antimicrobial agents against rapidly growing aerobic bacteria involved in infectious diseases
EUCAST (European Committeon Antimicrobial Susceptibility Testing) quality control table. Access link: https://www.eucast.org/ast_of_bacteria/quality_control/
EUCAST (European Committeon Antimicrobial Susceptibility Testing). EUCAST antifungal MIC method for yeasts.https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_7.3.2_Yeast_testing_definitive_revised_2020.pdf
Acknowledgements
Central Laboratory of Pharmacy, Faculty of Ankara University provided support for acquisition of the NMR, mass spectrometer and elemental analyzer used in this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Altaib, M., Doganc, F., Kaşkatepe, B. et al. Synthesis of some new 2-(substituted-phenyl)imidazo[4,5-c] and [4,5-b]pyridine derivatives and their antimicrobial activities. Mol Divers (2023). https://doi.org/10.1007/s11030-023-10715-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11030-023-10715-6