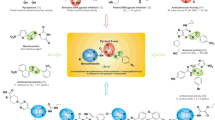
Abstract
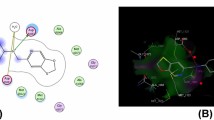
A general and sustainable approach for the synthesis of benzimidazole-thiazole compounds via an efficient, one-pot, domino, pseudo-four-component reaction using 5-amino-2-mercaptobenzimidazole, aralkyl halides, ammonium thiocyanate, and substituted α-bromo-acetophenones in glacial acetic acid at ambient temperature to give final compounds (4a–p) in good yields in shorter time. The spectral data of synthesized compounds were evaluated by analytical and spectral techniques (IR, 1H-NMR, 13C-NMR, and ESI-HRMS). Further, some of the synthesized compounds were screened for their in-vitro antibacterial activity studies using the agar well diffusion method against Gram-positive Streptococcus pneumoniae (2451) bacteria and Gram-negative Proteous mirabilis (2081) bacteria. Based on the MIC results, it was observed that the most active compounds 4b, 4e, 4f, and 4k show promising antibacterial activity with the zone of inhibition values of 2.85 cm 2.75 cm, 3.6 cm, and 3.3 cm against both Gram-negative and positive bacteria cell lines, respectively. Further, we have also insight into the molecular simulation studies, based on the binding results, compound 4i showed stable binding interactions with streptomycin drug with the active site of the gyrase protein (PDB ID: 1KIJ). The structure–activity relationship (SAR) studies of all the title scaffolds were also established. The antibacterial activity, molecular docking studies, and molecular dynamic simulations of the title compounds suggested that these are promising antibacterial active skeletons.
Graphical abstract











Similar content being viewed by others
References
Vila C, Rueping M (2013) Visible-light mediated heterogeneous C–H functionalization: oxidative multi-component reactions using a recyclable titanium dioxide (TiO2) catalyst. Green Chem 15:2056–2059. https://doi.org/10.1039/c3gc40587g
Mamidala S, Peddi SR, Aravilli RK, Jilloju PC, Manga V, Vedula RR (2020) Microwave irradiated one-pot, three-component synthesis of a new series of hybrid coumarin based thiazoles: antibacterial evaluation and molecular docking studies. J Mol Struct. https://doi.org/10.1016/j.molstruc.2020.129114
Vaarla K, Vishwapathi V, Vermeire K, Vedula RR, Kulkarni CV (2022) A facile one-pot multicomponent synthesis of alkyl 4-oxo-coumarinyl ethylidene hydrazono-thiazolidin-5-ylidene acetates and their antiviral activity. J Mol Struct 1249:131662. https://doi.org/10.1016/j.molstruc.2021.131662
Cascioferro S, Parrino B, Carbone D, Schillaci D, Giovannetti E, Cirrincione G, Diana P (2020) Thiazoles, their benzofused systems, and thiazolidinone derivatives: versatile and promising tools to combat antibiotic resistance. J Med Chem 63:7923–7956. https://doi.org/10.1021/acs.jmedchem.9b01245
Aggarwal T, Kumar S, Verma AK (2016) Iodine-mediated synthesis of heterocycles: via electrophilic cyclization of alkynes. Org Biomol Chem 14:7639–7653. https://doi.org/10.1039/c6ob01054g
Wang N, Saidhareddy P, Jiang X (2020) Construction of sulfur-containing moieties in the total synthesis of natural products. Nat Prod Rep 37:246–275. https://doi.org/10.1039/c8np00093j
Hassan A, Badr M, Hassan HA, Abdelhamid D, Abuo-Rahma GEA (2021) Novel 4-(piperazin-1-yl)quinolin-2(1H)-one bearing thiazoles with antiproliferative activity through VEGFR-2-TK inhibition. Bioorg Med Chem 40:116168. https://doi.org/10.1016/j.bmc.2021.116168
Osmaniye D, Görgülü S, Sağlık BN, Levent S, Özkay Y, Kaplancıklı ZA (2021) Design, synthesis, in vitro and in silico studies of some novel thiazole-dihydrofuran derivatives as aromatase inhibitors. Bioorg Chem 114:105123. https://doi.org/10.1016/j.bioorg.2021.105123
Bangade VM, Mali PR, Meshram HM (2021) Synthesis of potent anticancer substituted 5-benzimidazol-2-amino thiazoles controlled by bifunctional hydrogen bonding under microwave irradiations. J Org Chem 86:6056–6065. https://doi.org/10.1021/acs.joc.0c02542
Hu Y, Hu C, Pan G, Yu C, Ansari MF, Yadav Bheemanaboina RR, Cheng Y, Zhou C, Zhang J (2021) Novel chalcone-conjugated, multi-flexible end-group coumarin thiazole hybrids as potential antibacterial repressors against methicillin-resistant Staphylococcus aureus. Eur J Med Chem 222:113628. https://doi.org/10.1016/j.ejmech.2021.113628
Tomašič T, Katsamakas S, Hodnik Ž, Ilaš J, Brvar M, Solmajer T, Montalvão S, Tammela P, Banjanac M, Ergović G, Anderluh M, Mašič LP, Kikelj D (2015) Discovery of 4,5,6,7-tetrahydrobenzo[1,2-d ]thiazoles as novel DNA gyrase inhibitors targeting the ATP-binding site. J Med Chem 58:5501–5521. https://doi.org/10.1021/acs.jmedchem.5b00489
Yan Z, Liu A, Ou Y, Li J, Yi H, Zhang N, Liu M, Huang L, Ren J, Liu W, Hu A (2019) Design, synthesis and fungicidal activity evaluation of novel pyrimidinamine derivatives containing phenyl-thiazole/oxazole moiety. Bioorg Med Chem 27:3218–3228. https://doi.org/10.1016/j.bmc.2019.05.029
Adole VA, More RA, Jagdale BS, Pawar TB, Chobe SS (2020) Efficient synthesis, antibacterial, antifungal, antioxidant and cytotoxicity study of 2-(2-hydrazineyl)thiazole derivatives. ChemistrySelect 5:2778–2786. https://doi.org/10.1002/slct.201904609
Mayhoub AS, Khaliq M, Kuhn RJ, Cushman M (2011) Design, synthesis, and biological evaluation of thiazoles targeting flavivirus envelope proteins. J Med Chem 54:1704–1714. https://doi.org/10.1021/jm1013538
Sever B, Altıntop MD, Demir Y, Akalın Çiftçi G, Beydemir S, Özdemir A (2020) Design, synthesis, in vitro and in silico investigation of aldose reductase inhibitory effects of new thiazole-based compounds. Bioorg Chem 102:104110. https://doi.org/10.1016/j.bioorg.2020.104110
Zhang Z, Cao P, Fang M, Zou T, Han J, Duan Y, Xu H, Yang X, Li Q-S (2021) Design, synthesis, and SAR study of novel 4,5-dihydropyrazole-Thiazole derivatives with anti-inflammatory activities for the treatment of sepsis. Eur J Med Chem 225:113743. https://doi.org/10.1016/j.ejmech.2021.113743
Maghraby MTE, Abou-Ghadir OMF, Abdel-Moty SG, Ali AY, Salem OIA (2020) Novel class of benzimidazole-thiazole hybrids: the privileged scaffolds of potent anti-inflammatory activity with dual inhibition of cyclooxygenase and 15-lipoxygenase enzymes. Bioorg Med Chem 28:115403. https://doi.org/10.1016/j.bmc.2020.115403
Kiryanov AA, Sampson P, Seed AJ (2001) Synthesis of 2-alkoxy-substituted thiophenes, 1,3-thiazoles, and related S-heterocycles via Lawesson’s reagent-mediated cyclization under microwave irradiation: applications for liquid crystal synthesis. J Org Chem 66(23):7925–7929
Hodgetts KJ, Kershaw MT (2002) Regiocontrolled synthesis of substituted thiazoles. Org Lett 4:1363–1365. https://doi.org/10.1021/ol025688u
Cordeiro R, Kachroo M (2020) Synthesis and biological evaluation of anti-tubercular activity of Schiff bases of 2-amino thiazoles. Bioorg Med Chem Lett 30:127655. https://doi.org/10.1016/j.bmcl.2020.127655
Fayed EA, Ragab A, Ezz Eldin RR, Bayoumi AH, Ammar YA (2021) In vivo sreening 1 toxicity studies of indolinone incorporated thiosemicarbazone, thiazole and piperidinosulfonyl moieties as anticonvulsant agents. Bioorg Chem 116:105300. https://doi.org/10.1016/j.bioorg.2021.105300
El-Naggar AM, El-Hashash MA, Elkaeed EB (2021) Eco-friendly sequential one-pot synthesis, molecular docking, and anticancer evaluation of arylidene-hydrazinyl-thiazole derivatives as CDK2 inhibitors. Bioorg. Chem. 108:104615. https://doi.org/10.1016/j.bioorg.2020.104615
Alkhaldi AAM, Al-Sanea MM, Nocentini A, Eldehna WM, Elsayed ZM, Bonardi A, Abo-Ashour MF, El-Damasy AK, Abdel-Maksoud MS, Al-Warhi T, Gratteri P, Abdel-Aziz HA, Supuran CT, El-Haggar R (2020) 3-Methylthiazolo[3,2-a]benzimidazole-benzenesulfonamide conjugates as novel carbonic anhydrase inhibitors endowed with anticancer activity: design, synthesis, biological and molecular modeling studies. Eur J Med Chem 207:112745–46. https://doi.org/10.1016/j.ejmech.2020.112745
Kankala S, Kankala RK, Gundepaka P, Thota N, Nerella S, Gangula MR, Guguloth H, Kagga M, Vadde R, Vasam CS (2013) Regioselective synthesis of isoxazole–mercaptobenzimidazole hybrids and their in vivo analgesic and anti-inflammatory activity studies. Bioorg Med Chem Lett 23:1306–1309. https://doi.org/10.1016/j.bmcl.2012.12.101
Sharma R, Bali A, Chaudhari BB (2017) Synthesis of methanesulphonamido-benzimidazole derivatives as gastro-sparing antiinflammatory agents with antioxidant effect. Bioorg Med Chem Lett 27:3007–3013. https://doi.org/10.1016/j.bmcl.2017.05.017
Chaurasia H, Singh VK, Mishra R, Yadav AK, Ram NK, Singh P, Singh RK (2021) Molecular modelling, synthesis and antimicrobial evaluation of benzimidazole nucleoside mimetics. Bioorg Chem 115:105227. https://doi.org/10.1016/j.bioorg.2021.105227
Abdel-Motaal M, Almohawes K, Tantawy MA (2020) Antimicrobial evaluation and docking study of some new substituted benzimidazole-2yl derivatives. Bioorg Chem 101:103972. https://doi.org/10.1016/j.bioorg.2020.103972
Huo X, Hou D, Wang H, He B, Fang J, Meng Y, Liu L, Wei Z, Wang Z, Liu F-W (2021) Design, synthesis, in vitro and in vivo anti-respiratory syncytial virus (RSV) activity of novel oxizine fused benzimidazole derivatives. Eur J Med Chem 224:113684. https://doi.org/10.1016/j.ejmech.2021.113684
Zhou B, Li B, Yi W, Bu X, Ma L (2013) Synthesis, antioxidant, and antimicrobial evaluation of some 2-arylbenzimidazole derivatives. Bioorg Med Chem Lett 23:3759–3763. https://doi.org/10.1016/j.bmcl.2013.05.004
Galal SA, Abdelsamie AS, Shouman SA, Attia YM, Ali HI, Tabll A, El-Shenawy R, El Abd YS, Ali MM, Mahmoud AE, Abdel-Halim AH, Fyiad AA, Girgis AS, El-Diwani HI (2017) Part I: design, synthesis and biological evaluation of novel pyrazole-benzimidazole conjugates as checkpoint kinase 2 (Chk2) inhibitors with studying their activities alone and in combination with genotoxic drugs. Eur J Med Chem 134:392–405. https://doi.org/10.1016/j.ejmech.2017.03.090
Babkov DA, Zhukowskaya ON, Borisov AV, Babkova VA, Sokolova EV, Brigadirova AA, Litvinov RA, Kolodina AA, Morkovnik AS, Sochnev VS, Borodkin GS, Spasov AA (2019) Towards multi-target antidiabetic agents: discovery of biphenyl-benzimidazole conjugates as AMPK activators. Bioorg Med Chem Lett 29:2443–2447. https://doi.org/10.1016/j.bmcl.2019.07.035
Vitaku E, Smith DT, Njardarson JT (2014) Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J Med Chem 57:10257–10274. https://doi.org/10.1021/jm501100b
Jilloju PC, Persoons L, Kurapati SK et al (2022) Discovery of (±)-3-(1H-pyrazol-1-yl)-6,7-dihydro-5H-[1,2,4]triazolo[3,4-b][1,3,4] thiadiazine derivatives with promising in vitro anticoronavirus and antitumoral activity. Mol Divers 26:1357–1371. https://doi.org/10.1007/s11030-021-10258-8
Privalsky TM, Soohoo AM, Wang J, Walsh CT, Wright GD, Gordon EM, Gray NS, Khosla C (2021) Prospects for antibacterial discovery and development. J Am Chem Soc 143:21127–21142. https://doi.org/10.1021/jacs.1c10200
Lu J, Patel S, Sharma N, Soisson SM, Kishii R, Takei M, Fukuda Y, Lumb KJ, Singh SB (2014) Structures of kibdelomycin bound to Staphylococcus aureus GyrB and ParE showed a novel U-shaped binding mode. ACS Chem Biol 9:2023–2031. https://doi.org/10.1021/cb5001197
Arévalo JMC, Amorim JC (2022) Virtual screening, optimization and molecular dynamics analyses highlighting a pyrrolo[1,2-a]quinazoline derivative as a potential inhibitor of DNA gyrase B of Mycobacterium tuberculosis. Sci Rep 12:4742. https://doi.org/10.1038/s41598-022-08359-x
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461. https://doi.org/10.1002/jcc.21334
Salentin S, Schreiber S, Haupt VJ, Adasme MF, Schroeder M (2015) PLIP: fully automated protein–ligand interaction profiler. Nucleic Acids Res 43:W443–W447. https://doi.org/10.1093/nar/gkv315
Van Der Spoel D, Lindahl E, Hess B, Groenhof G, Mark AE, Berendsen HJC (2005) GROMACS: fast, flexible, and free. J Comput Chem 26:1701–1718. https://doi.org/10.1002/jcc.20291
Robertson MJ, Tirado-Rives J, Jorgensen WL (2015) Improved peptide and protein torsional energetics with the OPLS-AA force field. J Chem Theory Comput 11:3499–3509. https://doi.org/10.1021/acs.jctc.5b00356
Acknowledgements
The authors are thankful to the Director of the National Institute of Technology, Warangal Telangana, India, for providing lab facilities, and one of the authors R.C. thankful to the Ministry of Education, Government of India, for providing a research fellowship. R. C. wishes to thank Dr. Amrutha V Audipudi for biological activity and computational calculations.
Author information
Authors and Affiliations
Contributions
Raju Chedupaka carried out the laboratory work, conceptualization, wrote the main manuscript text, and methodology. Amrutha V Audipudi did the work on biology part of the manuscript. Akanksha Ashok Sangolkar did the computational part of the manuscript. Srikanth Mamidala helped in reviewing, editing, and formal analysis of the manuscript. Papisetti Venkatesham and Santhosh Penta helped for validation. Rajeswar Rao Vedula was the supervisor of the research work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MPG 30432 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chedupaka, R., Audipudi, A.V., Sangolkar, A.A. et al. Design, synthesis, molecular docking, and dynamic studies of novel thiazole derivatives incorporating benzimidazole moiety and assessment as antibacterial agents. Mol Divers (2023). https://doi.org/10.1007/s11030-023-10675-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11030-023-10675-x