Abstract
CNS disorders are indications with a very high unmet medical needs, relatively smaller number of available drugs, and a subpar satisfaction level among patients and caregiver. Discovery of CNS drugs is extremely expensive affair with its own unique challenges leading to extremely high attrition rates and low efficiency. With explosion of data in information age, there is hardly any aspect of life that has not been touched by data driven technologies such as artificial intelligence (AI) and machine learning (ML). Drug discovery is no exception, emergence of big data via genomic, proteomic, biological, and chemical technologies has driven pharmaceutical giants to collaborate with AI oriented companies to revolutionise drug discovery, with the goal of increasing the efficiency of the process. In recent years many examples of innovative applications of AI and ML techniques in CNS drug discovery has been reported. Research on therapeutics for diseases such as schizophrenia, Alzheimer’s and Parkinsonism has been provided with a new direction and thrust from these developments. AI and ML has been applied to both ligand-based and structure-based drug discovery and design of CNS therapeutics. In this review, we have summarised the general aspects of AI and ML from the perspective of drug discovery followed by a comprehensive coverage of the recent developments in the applications of AI/ML techniques in CNS drug discovery.
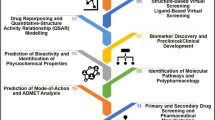
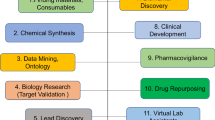
Graphical abstract







Similar content being viewed by others
References
DiMasi JA, Grabowski HG, Hansen RW (2015) The cost of drug development. N Engl J Med 372(20):1972. https://doi.org/10.1056/NEJMc1504317
Morgan S et al (2011) The cost of drug development: a systematic review. Health Policy 100(1):4–17. https://doi.org/10.1016/j.healthpol.2010.12.002
Paul SM et al (2010) How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat Rev Drug Discov 9(3):203–214. https://doi.org/10.1038/nrd3078
Cacace E, Kritikos G, Typas A (2017) Chemical genetics in drug discovery. Curr Opin Syst Biol 4:35–42. https://doi.org/10.1016/j.coisb.2017.05.020
Chan CY et al (2013) Accelerating drug discovery via organs-on-chips. Lab Chip 13(24):4697–4710. https://doi.org/10.1039/c3lc90115g
Matthews H, Hanison J, Nirmalan N (2016) “Omics”-informed drug and biomarker discovery: opportunities, challenges and future perspectives. Proteomes. https://doi.org/10.3390/proteomes4030028
Schenone M et al (2013) Target identification and mechanism of action in chemical biology and drug discovery. Nat Chem Biol 9(4):232–240. https://doi.org/10.1038/nchembio.1199
Santos R et al (2017) A comprehensive map of molecular drug targets. Nat Rev Drug Discov 16(1):19–34. https://doi.org/10.1038/nrd.2016.230
Rask-Andersen M, Almén MS, Schiöth HB (2011) Trends in the exploitation of novel drug targets. Nat Rev Drug Discov 10(8):579–590. https://doi.org/10.1038/nrd3478
Mohs RC, Greig NH (2017) Drug discovery and development: role of basic biological research. Alzheimers Dement (N Y) 3(4):651–657. https://doi.org/10.1016/j.trci.2017.10.005
Gribkoff VK, Kaczmarek LK (2017) The need for new approaches in CNS drug discovery: why drugs have failed, and what can be done to improve outcomes. Neuropharmacology 120:11–19. https://doi.org/10.1016/j.neuropharm.2016.03.021
Abbott NJ (2013) Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis 36(3):437–449. https://doi.org/10.1007/s10545-013-9608-0
Pankevich DE et al (2014) Improving and accelerating drug development for nervous system disorders. Neuron 84(3):546–553. https://doi.org/10.1016/j.neuron.2014.10.007
de Lange ECM et al (2017) Novel CNS drug discovery and development approach: model-based integration to predict neuro-pharmacokinetics and pharmacodynamics. Expert Opin Drug Discov 12(12):1207–1218. https://doi.org/10.1080/17460441.2017.1380623
Chan HCS et al (2019) Advancing drug discovery via artificial intelligence. Trends Pharmacol Sci 40(8):592–604. https://doi.org/10.1016/j.tips.2019.06.004
Smith JS, Roitberg AE, Isayev O (2018) Transforming computational drug discovery with machine learning and AI. ACS Med Chem Lett 9(11):1065–1069. https://doi.org/10.1021/acsmedchemlett.8b00437
Zhavoronkov A (2018) Artificial intelligence for drug discovery, biomarker development, and generation of novel chemistry. Mol Pharm 15(10):4311–4313. https://doi.org/10.1021/acs.molpharmaceut.8b00930
Mak K-K, Pichika MR (2019) Artificial intelligence in drug development: present status and future prospects. Drug Discov Today 24(3):773–780. https://doi.org/10.1016/j.drudis.2018.11.014
Smalley E (2017) AI-powered drug discovery captures pharma interest. Nat Biotechnol 35:604
Elbadawi M, Gaisford S, Basit AW (2021) Advanced machine-learning techniques in drug discovery. Drug Discov Today 26(3):769–777. https://doi.org/10.1016/j.drudis.2020.12.003
Zhu H (2020) Big data and artificial intelligence modeling for drug discovery. Annu Rev Pharmacol Toxicol 60(1):573–589. https://doi.org/10.1146/annurev-pharmtox-010919-023324
Schadt EE et al (2011) Cloud and heterogeneous computing solutions exist today for the emerging big data problems in biology. Nat Rev Genet 12(3):224–224. https://doi.org/10.1038/nrg2857-c2
Marx V (2013) The big challenges of big data. Nature 498(7453):255–260. https://doi.org/10.1038/498255a
Brown N et al (2018) Big data in drug discovery. In: Witty DR, Cox B (eds) Progress in medicinal chemistry. Elsevier, New York, pp 277–356. https://doi.org/10.1016/bs.pmch.2017.12.003
Liu R, Li X, Lam KS (2017) Combinatorial chemistry in drug discovery. Curr Opin Chem Biol 38:117–126. https://doi.org/10.1016/j.cbpa.2017.03.017
Benz M et al (2019) Marrying chemistry with biology by combining on-chip solution-based combinatorial synthesis and cellular screening. Nat Commun 10(1):2879. https://doi.org/10.1038/s41467-019-10685-0
Borrel A et al (2020) High-throughput screening to predict chemical-assay interference. Sci Rep 10(1):3986–3986. https://doi.org/10.1038/s41598-020-60747-3
Broach JR, Thorner J (1996) High-throughput screening for drug discovery. Nature 384(6604 Suppl):14–16. https://doi.org/10.1038/384014a0
Zhu H et al (2014) Big data in chemical toxicity research: the use of high-throughput screening assays to identify potential toxicants. Chem Res Toxicol 27(10):1643–1651. https://doi.org/10.1021/tx500145h
Macarron R et al (2011) Impact of high-throughput screening in biomedical research. Nat Rev Drug Discov 10(3):188–195. https://doi.org/10.1038/nrd3368
Klekota J et al (2006) Using high-throughput screening data to discriminate compounds with single-target effects from those with side effects. J Chem Inf Model 46(4):1549–1562. https://doi.org/10.1021/ci050495h
Favaretto M et al (2020) What is your definition of Big Data? Researchers’ understanding of the phenomenon of the decade. PLoS ONE 15(2):e0228987. https://doi.org/10.1371/journal.pone.0228987
Younas M (2019) Research challenges of big data. SOCA 13(2):105–107. https://doi.org/10.1007/s11761-019-00265-x
Ishwarappa AJ (2015) A brief introduction on big data 5Vs characteristics and Hadoop technology. Procedia Comput Sci 48:319–324. https://doi.org/10.1016/j.procs.2015.04.188
Leonelli S (2019) The challenges of big data biology. Elife 8:e47381. https://doi.org/10.7554/eLife.47381
Lee CH, Yoon HJ (2017) Medical big data: promise and challenges. Kidney Res Clin Pract 36(1):3–11. https://doi.org/10.23876/j.krcp.2017.36.1.3
Scheeder C, Heigwer F, Boutros M (2018) Machine learning and image-based profiling in drug discovery. Curr Opin Syst Biol 10:43–52. https://doi.org/10.1016/j.coisb.2018.05.004
Korotcov A et al (2017) Comparison of deep learning with multiple machine learning methods and metrics using diverse drug discovery data sets. Mol Pharm 14(12):4462–4475. https://doi.org/10.1021/acs.molpharmaceut.7b00578
Jing Y et al (2018) Deep learning for drug design: an artificial intelligence paradigm for drug discovery in the big data era. AAPS J 20(3):58. https://doi.org/10.1208/s12248-018-0210-0
Wooller SK et al (2017) Bioinformatics in translational drug discovery. Biosci Rep. https://doi.org/10.1042/bsr20160180
Batool M, Ahmad B, Choi S (2019) A structure-based drug discovery paradigm. Int J Mol Sci. https://doi.org/10.3390/ijms20112783
Liu B et al (2019) Artificial intelligence and big data facilitated targeted drug discovery. Stroke Vasc Neurol 4(4):206–213. https://doi.org/10.1136/svn-2019-000290
Zhao L et al (2020) Advancing computer-aided drug discovery (CADD) by big data and data-driven machine learning modeling. Drug Discov Today 25(9):1624–1638. https://doi.org/10.1016/j.drudis.2020.07.005
Patel L et al (2020) Machine learning methods in drug discovery. Molecules. https://doi.org/10.3390/molecules25225277
Glicksberg BS et al (2019) Leveraging big data to transform drug discovery. Methods Mol Biol 1939:91–118. https://doi.org/10.1007/978-1-4939-9089-4_6
Chen X et al (2016) Drug-target interaction prediction: databases, web servers and computational models. Brief Bioinform 17(4):696–712. https://doi.org/10.1093/bib/bbv066
Meier K et al (2020) The generated databases (GDBs) as a source of 3D-shaped building blocks for use in medicinal chemistry and drug discovery. Chimia (Aarau) 74(4):241–246. https://doi.org/10.2533/chimia.2020.241
Xie T et al (2015) Review of natural product databases. Cell Prolif 48(4):398–404. https://doi.org/10.1111/cpr.12190
Nguyen-Vo TH et al (2020) Plant metabolite databases: from herbal medicines to modern drug discovery. J Chem Inf Model 60(3):1101–1110. https://doi.org/10.1021/acs.jcim.9b00826
Fuentes G, Oyarzabal J, Rojas AM (2009) Databases of protein-protein interactions and their use in drug discovery. Curr Opin Drug Discov Devel 12(3):358–366
Yang B et al (2019) Computer-assisted drug virtual screening based on the natural product databases. Curr Pharm Biotechnol 20(4):293–301. https://doi.org/10.2174/1389201020666190328115411
Potemkin V, Potemkin A, Grishina M (2018) Internet resources for drug discovery and design. Curr Top Med Chem 18(22):1955–1975. https://doi.org/10.2174/1568026619666181129142127
Kim S (2016) Getting the most out of PubChem for virtual screening. Expert Opin Drug Discov 11(9):843–855. https://doi.org/10.1080/17460441.2016.1216967
Kim S et al (2018) Finding potential multitarget ligands using PubChem. Methods Mol Biol 1825:63–91. https://doi.org/10.1007/978-1-4939-8639-2_2
Wang Y et al (2012) PubChem’s bioassay database. Nucleic Acids Res 40(Database issue):D400–D412. https://doi.org/10.1093/nar/gkr1132
Kim S et al (2021) PubChem in 2021: new data content and improved web interfaces. Nucleic Acids Res 49(D1):D1388-d1395. https://doi.org/10.1093/nar/gkaa971
Kim S et al (2016) Literature information in PubChem: associations between PubChem records and scientific articles. J Cheminform 8:32. https://doi.org/10.1186/s13321-016-0142-6
Umesh PK, Dubey VK (2021) Virtual screening and repurposing of FDA-approved drugs from ZINC database to identify potential autophagy inhibitors exploiting autophagy related 4A cysteine peptidase as a target: potential as novel anti-cancer molecule. J Biomol Struct Dyn 2021:1–17. https://doi.org/10.1080/07391102.2020.1869100
Boucherit H et al (2020) The research of new inhibitors of bacterial methionine aminopeptidase by structure based virtual screening approach of zinc database and in vitro validation. Curr Comput Aided Drug Des 16(4):389–401. https://doi.org/10.2174/1573409915666190617165643
Abdusalam AAA, Murugaiyah V (2020) Identification of potential inhibitors of 3CL protease of SARS-CoV-2 from zinc database by molecular docking-based virtual screening. Front Mol Biosci 7:603037. https://doi.org/10.3389/fmolb.2020.603037
Awale M, Jin X, Reymond JL (2015) Stereoselective virtual screening of the ZINC database using atom pair 3D-fingerprints. J Cheminform 7:3. https://doi.org/10.1186/s13321-014-0051-5
Monika KJ, Singh K (2013) Virtual screening using the ligand ZINC database for novel lipoxygenase-3 inhibitors. Bioinformation 9(11):583–587. https://doi.org/10.6026/97320630009583
Nogara PA et al (2015) Virtual screening of acetylcholinesterase inhibitors using the Lipinski’s rule of five and ZINC databank. Biomed Res Int 2015:870389. https://doi.org/10.1155/2015/870389
Irwin JJ (2008) Using ZINC to acquire a virtual screening library. Curr Protoc Bioinformatics. https://doi.org/10.1002/0471250953.bi1406s22
Sterling T, Irwin JJ (2015) ZINC 15—ligand discovery for everyone. J Chem Inf Model 55(11):2324–2337. https://doi.org/10.1021/acs.jcim.5b00559
Real Compound Libraries (2021) Enamine. https://enamine.net/library-synthesis/real-compounds/real-compound-libraries
Klingler F-M et al (2019) SAR by space: enriching hit sets from the chemical space. Molecules. https://doi.org/10.3390/molecules24173096
Grygorenko OO et al (2020) Generating multibillion chemical space of readily accessible screening compounds. iScience 23(11):101681. https://doi.org/10.1016/j.isci.2020.101681
Sorokina M et al (2021) COCONUT online: collection of open natural products database. J Cheminf 13(1):2. https://doi.org/10.1186/s13321-020-00478-9
Davies M et al (2015) ChEMBL web services: streamlining access to drug discovery data and utilities. Nucleic Acids Res 43(W1):W612–W620. https://doi.org/10.1093/nar/gkv352
Papadatos G, Overington JP (2014) The ChEMBL database: a taster for medicinal chemists. Future Med Chem 6(4):361–364. https://doi.org/10.4155/fmc.14.8
Capecchi A et al (2019) PubChem and ChEMBL beyond Lipinski. Mol Inform 38(5):e1900016. https://doi.org/10.1002/minf.201900016
Mendez D et al (2019) ChEMBL: towards direct deposition of bioassay data. Nucleic Acids Res 47(D1):D930–D940. https://doi.org/10.1093/nar/gky1075
Papadatos G et al (2016) SureChEMBL: a large-scale, chemically annotated patent document database. Nucleic Acids Res 44(D1):D1220–D1228. https://doi.org/10.1093/nar/gkv1253
Falaguera MJ, Mestres J (2021) Identification of the core chemical structure in SureChEMBL patents. J Chem Inf Model 61(5):2241–2247. https://doi.org/10.1021/acs.jcim.1c00151
Wirth M et al (2013) SwissBioisostere: a database of molecular replacements for ligand design. Nucleic Acids Res 41(Database issue):D1137–D1143. https://doi.org/10.1093/nar/gks1059
Daina A, Zoete V (2019) Application of the SwissDrugDesign online resources in virtual screening. Int J Mol Sci. https://doi.org/10.3390/ijms20184612
Zoete V et al (2016) SwissSimilarity: a web tool for low to ultra high throughput ligand-based virtual screening. J Chem Inf Model 56(8):1399–1404. https://doi.org/10.1021/acs.jcim.6b00174
Cole JC et al (2018) Knowledge-based conformer generation using the Cambridge structural database. J Chem Inf Model 58(3):615–629. https://doi.org/10.1021/acs.jcim.7b00697
Groom CR et al (2016) The Cambridge structural database. Acta Crystallogr B Struct Sci Cryst Eng Mater 72(Pt 2):171–179. https://doi.org/10.1107/s2052520616003954
Wishart DS et al (2008) DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res 36(1):D901–D906. https://doi.org/10.1093/nar/gkm958
Wishart DS et al (2006) DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res 34(Database issue):D668–D672. https://doi.org/10.1093/nar/gkj067
Wishart DS et al (2018) DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res 46(D1):D1074–D1082. https://doi.org/10.1093/nar/gkx1037
Wishart DS, Wu A (2016) Using DrugBank for in silico drug exploration and discovery. Curr Protoc Bioinformatics 54:14.4.1-14.4.31. https://doi.org/10.1002/cpbi.1
Wishart DS (2008) DrugBank and its relevance to pharmacogenomics. Pharmacogenomics 9(8):1155–1162. https://doi.org/10.2217/14622416.9.8.1155
Berman HM et al (2000) The protein data bank. Nucleic Acids Res 28(1):235–242. https://doi.org/10.1093/nar/28.1.235
Burley SK et al (2017) Protein data bank (PDB): the single global macromolecular structure archive. Methods Mol Biol 1607:627–641. https://doi.org/10.1007/978-1-4939-7000-1_26
Prestegard JH (2021) A perspective on the PDB’s impact on the field of glycobiology. J Biol Chem 296:100556. https://doi.org/10.1016/j.jbc.2021.100556
Jiménez J et al (2017) DeepSite: protein-binding site predictor using 3D-convolutional neural networks. Bioinformatics 33(19):3036–3042. https://doi.org/10.1093/bioinformatics/btx350
Fernández A (2019) Deep learning to therapeutically target unreported complexes. Trends Pharmacol Sci 40(8):551–554. https://doi.org/10.1016/j.tips.2019.04.009
Liñares-Blanco J et al (2020) Molecular docking and machine learning analysis of Abemaciclib in colon cancer. BMC Mol Cell Biol 21(1):52. https://doi.org/10.1186/s12860-020-00295-w
The UniProt Consortium (2019) UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res 47(D1):D506–D515. https://doi.org/10.1093/nar/gky1049
Sarker IH (2021) Machine learning: algorithms, real-world applications and research directions. SN Comput Sci 2(3):160. https://doi.org/10.1007/s42979-021-00592-x
The UniProt Consortium (2017) UniProt: the universal protein knowledgebase. Nucleic Acids Res 45(D1):D158-d169. https://doi.org/10.1093/nar/gkw1099
Pundir S, Martin MJ, O’Donovan C (2017) UniProt protein knowledgebase. Methods Mol Biol 1558:41–55. https://doi.org/10.1007/978-1-4939-6783-4_2
Wang Y et al (2020) Therapeutic target database 2020: enriched resource for facilitating research and early development of targeted therapeutics. Nucleic Acids Res 48(D1):D1031–D1041. https://doi.org/10.1093/nar/gkz981
Chen X, Ji ZL, Chen YZ (2002) TTD: therapeutic target database. Nucleic Acids Res 30(1):412–415. https://doi.org/10.1093/nar/30.1.412
Hamet P, Tremblay J (2017) Artificial intelligence in medicine. Metabolism 69:S36–S40. https://doi.org/10.1016/j.metabol.2017.01.011
Turing AM (1950) I—Computing machinery and intelligence. Mind LIX(236):433–460. https://doi.org/10.1093/mind/LIX.236.433
Carbonell JG, Michalski RS, Mitchell TM (1983) An overview of machine learning. In: Michalski RS, Carbonell JG, Mitchell TM (eds) Machine learning: an artificial intelligence approach. Springer, Berlin, pp 3–23. https://doi.org/10.1007/978-3-662-12405-5_1
Todeschini R, Consonni V (2000) Frontmatter. In: Handbook of molecular descriptors. Wiley, Weinheim, pp i–xxi. https://doi.org/10.1002/9783527613106.fmatter
Dong J et al (2015) ChemDes: an integrated web-based platform for molecular descriptor and fingerprint computation. J Cheminf 7(1):60. https://doi.org/10.1186/s13321-015-0109-z
Sukumar N et al (2011) Molecular descriptors for biological systems. In: Guha R, Bender A (eds) Computational approaches in cheminformatics and bioinformatics. Wiley-VCH, Weinheim, pp 107–143. https://doi.org/10.1002/9781118131411.ch5
Todeschini R, Consonni V, Gramatica P (2009) Chemometrics in QSAR. In: Brown SD, Tauler R, Walczak B (eds) Comprehensive chemometrics. Elsevier, Oxford, pp 129–172. https://doi.org/10.1016/B978-044452701-1.00007-7
Bajorath J (2001) Selected concepts and investigations in compound classification, molecular descriptor analysis, and virtual screening. J Chem Inf Comput Sci 41(2):233–245. https://doi.org/10.1021/ci0001482
Raymond JW, Willett P (2002) Effectiveness of graph-based and fingerprint-based similarity measures for virtual screening of 2D chemical structure databases. J Comput Aided Mol Des 16(1):59–71. https://doi.org/10.1023/A:1016387816342
Ivanciuc O (2013) Chemical graphs, molecular matrices and topological indices in chemoinformatics and quantitative structure-activity relationships. Curr Comput Aided Drug Des 9(2):153–163. https://doi.org/10.2174/1573409911309020002
Kombo DC et al (2013) 3D molecular descriptors important for clinical success. J Chem Inf Model 53(2):327–342. https://doi.org/10.1021/ci300445e
Orosz Á, Héberger K, Rácz A (2022) Comparison of descriptor-and fingerprint sets in machine learning models for ADME-Tox targets. Front Chem 10:852893. https://doi.org/10.3389/fchem.2022.852893
Senese CL et al (2004) 4D-fingerprints, universal QSAR and QSPR descriptors. J Chem Inf Comput Sci 44(5):1526–1539. https://doi.org/10.1021/ci049898s
Jaroslaw P (2009) Receptor dependent multidimensional QSAR for modeling drug–receptor interactions. Curr Med Chem 16(25):3243–3257. https://doi.org/10.2174/092986709788803286
Hayakawa D et al (2020) A molecular interaction field describing nonconventional intermolecular interactions and its application to protein–ligand interaction prediction. J Mol Graph Model 96:107515. https://doi.org/10.1016/j.jmgm.2019.107515
Chartier M, Najmanovich R (2015) Detection of binding site molecular interaction field similarities. J Chem Inf Model 55(8):1600–1615. https://doi.org/10.1021/acs.jcim.5b00333
Artese A et al (2013) Molecular interaction fields in drug discovery: recent advances and future perspectives. WIREs Comput Mol Sci 3(6):594–613. https://doi.org/10.1002/wcms.1150
Ranade V (2006) Molecular interaction fields. Am J Therapeutics 13(4):385–386
Bertoni M et al (2021) Bioactivity descriptors for uncharacterized chemical compounds. Nat Commun 12(1):3932. https://doi.org/10.1038/s41467-021-24150-4
Chuang KV, Gunsalus LM, Keiser MJ (2020) Learning molecular representations for medicinal chemistry. J Med Chem 63(16):8705–8722. https://doi.org/10.1021/acs.jmedchem.0c00385
Xue L, Bajorath J (2000) Molecular descriptors in chemoinformatics, computational combinatorial chemistry, and virtual screening. Comb Chem High Throughput Screen 3(5):363–372. https://doi.org/10.2174/1386207003331454
Rogers D, Hahn M (2010) Extended-connectivity fingerprints. J Chem Inf Model 50(5):742–754. https://doi.org/10.1021/ci100050t
Glem RC et al (2006) Circular fingerprints: flexible molecular descriptors with applications from physical chemistry to ADME. IDrugs 9(3):199–204
Kearnes S et al (2016) Molecular graph convolutions: moving beyond fingerprints. J Comput Aided Mol Des 30(8):595–608. https://doi.org/10.1007/s10822-016-9938-8
Wang X et al (2019) Molecule property prediction based on spatial graph embedding. J Chem Inf Model 59(9):3817–3828. https://doi.org/10.1021/acs.jcim.9b00410
Steinbeck C et al (2006) Recent developments of the chemistry development kit (CDK)—an open-source java library for chemo- and bioinformatics. Curr Pharm Des 12(17):2111–2120. https://doi.org/10.2174/138161206777585274
Willighagen EL et al (2017) The Chemistry Development Kit (CDK) v2.0: atom typing, depiction, molecular formulas, and substructure searching. J Cheminform 9(1):33. https://doi.org/10.1186/s13321-017-0220-4
Lovrić M, Molero JM, Kern R (2019) PySpark and RDKit: moving towards big data in cheminformatics. Mol Inform 38(6):e1800082. https://doi.org/10.1002/minf.201800082
Tangadpalliwar SR et al (2019) ChemSuite: a package for chemoinformatics calculations and machine learning. Chem Biol Drug Des 93(5):960–964. https://doi.org/10.1111/cbdd.13479
Chen Z et al (2018) iFeature: a Python package and web server for features extraction and selection from protein and peptide sequences. Bioinformatics 34(14):2499–2502. https://doi.org/10.1093/bioinformatics/bty140
Taguchi YH, Turki T (2020) A new advanced in silico drug discovery method for novel coronavirus (SARS-CoV-2) with tensor decomposition-based unsupervised feature extraction. PLoS ONE 15(9):e0238907. https://doi.org/10.1371/journal.pone.0238907
Ma’ayan A et al (2007) Network analysis of FDA approved drugs and their targets. Mt Sinai J Med N Y 74(1):27–32. https://doi.org/10.1002/msj.20002
Sarkans U et al (2021) From ArrayExpress to BioStudies. Nucleic Acids Res 49(D1):D1502–D1506. https://doi.org/10.1093/nar/gkaa1062
Clough E, Barrett T (2016) The gene expression omnibus database. Methods Mol Biol (Clifton, N.J.) 1418:93–110. https://doi.org/10.1007/978-1-4939-3578-9_5
Parkinson H et al (2007) ArrayExpress—a public database of microarray experiments and gene expression profiles. Nucleic Acids Res 35(Database issue):D747–D750. https://doi.org/10.1093/nar/gkl995
Athar A et al (2019) ArrayExpress update—from bulk to single-cell expression data. Nucleic Acids Res 47(D1):D711–D715. https://doi.org/10.1093/nar/gky964
Cao C, Moult J (2014) GWAS and drug targets. BMC Genomics 15(Suppl 4):S5. https://doi.org/10.1186/1471-2164-15-S4-S5
Beck T, Shorter T, Brookes AJ (2020) GWAS central: a comprehensive resource for the discovery and comparison of genotype and phenotype data from genome-wide association studies. Nucleic Acids Res 48(D1):D933–D940. https://doi.org/10.1093/nar/gkz895
Buniello A et al (2019) The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res 47(D1):D1005–D1012. https://doi.org/10.1093/nar/gky1120
Wei J et al (2021) Genome-wide CRISPR screens reveal host factors critical for SARS-CoV-2 infection. Cell 184(1):76–91. https://doi.org/10.1016/j.cell.2020.10.028
King EA, Davis JW, Degner JF (2019) Are drug targets with genetic support twice as likely to be approved? Revised estimates of the impact of genetic support for drug mechanisms on the probability of drug approval. PLoS Genet 15(12):e1008489. https://doi.org/10.1371/journal.pgen.1008489
Kodama Y et al (2012) The Sequence Read Archive: explosive growth of sequencing data. Nucleic Acids Res 40(Database issue):D54–D56. https://doi.org/10.1093/nar/gkr854
Han Y et al (2019) DriverML: a machine learning algorithm for identifying driver genes in cancer sequencing studies. Nucleic Acids Res 47(8):e45. https://doi.org/10.1093/nar/gkz096
Han Y et al (2021) Corrigendum to article “DriverML: a machine learning algorithm for identifying driver genes in cancer sequencing studies.” Nucleic Acids Res 49(7):4196. https://doi.org/10.1093/nar/gkab193
Dhasmana A et al (2020) Topological and system-level protein interaction network (PIN) analyses to deduce molecular mechanism of curcumin. Sci Rep 10(1):12045. https://doi.org/10.1038/s41598-020-69011-0
Zhang Z et al (2018) Resurrected protein interaction networks reveal the innovation potential of ancient whole-genome duplication. Plant Cell 30(11):2741–2760. https://doi.org/10.1105/tpc.18.00409
Farooq QUA et al (2020) A systems biology-driven approach to construct a comprehensive protein interaction network of influenza A virus with its host. BMC Infect Dis 20(1):480. https://doi.org/10.1186/s12879-020-05214-0
Han L et al (2017) Human enterovirus 71 protein interaction network prompts antiviral drug repositioning. Sci Rep 7:43143. https://doi.org/10.1038/srep43143
Farooq QUA et al (2020) Inferring Virus-Host relationship between HPV and its host Homo sapiens using protein interaction network. Sci Rep 10(1):8719. https://doi.org/10.1038/s41598-020-65837-w
Hase T et al (2009) Structure of protein interaction networks and their implications on drug design. PLOS Comput Biol 5(10):e1000550. https://doi.org/10.1371/journal.pcbi.1000550
Tsuji S et al (2021) Artificial intelligence-based computational framework for drug-target prioritization and inference of novel repositionable drugs for Alzheimer’s disease. Alzheimer’s Res Therapy 13(1):92. https://doi.org/10.1186/s13195-021-00826-3
iCLUE&ASK (2021) https://icluenask.standigm.com/about
White J (2020) PubMed. Med Ref Serv Q 39(4):382–387. https://doi.org/10.1080/02763869.2020.1826228
Kim J et al (2013) DigSee: disease gene search engine with evidence sentences (version cancer). Nucleic Acids Res 41(Web Server Issue):W510–W517. https://doi.org/10.1093/nar/gkt531
Nayal M, Honig B (2006) On the nature of cavities on protein surfaces: application to the identification of drug-binding sites. Proteins 63(4):892–906. https://doi.org/10.1002/prot.20897
Kandoi G, Acencio ML, Lemke N (2015) Prediction of druggable proteins using machine learning and systems biology: A mini-review. Front Physiol 6:366. https://doi.org/10.3389/fphys.2015.00366
Rifaioglu AS et al (2021) MDeePred: novel multi-channel protein featurization for deep learning-based binding affinity prediction in drug discovery. Bioinformatics 37(5):693–704. https://doi.org/10.1093/bioinformatics/btaa858
Kandel J, Tayara H, Chong KT (2021) PUResNet: prediction of protein–ligand binding sites using deep residual neural network. J Cheminf 13(1):65. https://doi.org/10.1186/s13321-021-00547-7
Yuan J-H et al (2020) Druggability assessment in TRAPP using machine learning approaches. J Chem Inf Model 60(3):1685–1699. https://doi.org/10.1021/acs.jcim.9b01185
Olah M et al (2005) WOMBAT: world of molecular bioactivity. Chemoinformatics Drug Discov 2005:221–239. https://doi.org/10.1002/3527603743.ch9
Lee K, Lee M, Kim D (2017) Utilizing random forest QSAR models with optimized parameters for target identification and its application to target-fishing server. BMC Bioinformatics 18(Suppl 16):567. https://doi.org/10.1186/s12859-017-1960-x
Senior AW et al (2020) Improved protein structure prediction using potentials from deep learning. Nature 577(7792):706–710. https://doi.org/10.1038/s41586-019-1923-7
Cyclica Launches Ligand Express™, a Disruptive Cloud-Based Platform to Revolutionize Drug Discovery (2017). https://www.cyclicarx.com/press-releases/cyclica-launches-ligand-express-a-disruptive-cloud-based-platform-to-revolutionize-drug-discovery
Korkmaz S, Zararsiz G, Goksuluk D (2015) MLViS: a web tool for machine learning-based virtual screening in early-phase of drug discovery and development. PLoS ONE 10(4):e0124600. https://doi.org/10.1371/journal.pone.0124600
Wójcikowski M, Zielenkiewicz P, Siedlecki P (2015) Open Drug Discovery Toolkit (ODDT): a new open-source player in the drug discovery field. J Cheminf 7(1):26. https://doi.org/10.1186/s13321-015-0078-2
Blaschke T et al (2020) REINVENT 2.0: an AI tool for de novo drug design. J Chem Inf Model 60(12):5918–5922. https://doi.org/10.1021/acs.jcim.0c00915
Alley EC et al (2019) Unified rational protein engineering with sequence-based deep representation learning. Nat Methods 16(12):1315–1322. https://doi.org/10.1038/s41592-019-0598-1
Amendola G, Cosconati S (2021) PyRMD: a new fully automated AI-powered ligand-based virtual screening tool. J Chem Inf Model 61(8):3835–3845. https://doi.org/10.1021/acs.jcim.1c00653
Gentile F et al (2020) Deep docking: a deep learning platform for augmentation of structure based drug discovery. ACS Cent Sci 6(6):939–949. https://doi.org/10.1021/acscentsci.0c00229
Bai Q et al (2020) MolAICal: a soft tool for 3D drug design of protein targets by artificial intelligence and classical algorithm. Brief Bioinformatics. https://doi.org/10.1093/bib/bbaa161
Yan Y et al (2017) Protein–ligand empirical interaction components for virtual screening. J Chem Inf Model 57(8):1793–1806. https://doi.org/10.1021/acs.jcim.7b00017
Cherkasov A et al (2009) Use of artificial intelligence in the design of small peptide antibiotics effective against a broad spectrum of highly antibiotic-resistant superbugs. ACS Chem Biol 4(1):65–74. https://doi.org/10.1021/cb800240j
Kinnings SL et al (2011) A machine learning-based method to improve docking scoring functions and its application to drug repurposing. J Chem Inf Model 51(2):408–419. https://doi.org/10.1021/ci100369f
Leong MK et al (2017) Prediction of N-methyl-d-aspartate receptor GluN1-ligand binding affinity by a novel SVM-pose/SVM-score combinatorial ensemble docking scheme. Sci Rep 7:40053. https://doi.org/10.1038/srep40053
Li H et al (2015) Improving AutoDock Vina using random forest: the growing accuracy of binding affinity prediction by the effective exploitation of larger data sets. Mol Inf 34(2–3):115–126. https://doi.org/10.1002/minf.201400132
Arciniega M, Lange OF (2014) Improvement of virtual screening results by docking data feature analysis. J Chem Inf Model 54(5):1401–1411. https://doi.org/10.1021/ci500028u
Waszkowycz B (2008) Towards improving compound selection in structure-based virtual screening. Drug Discov Today 13(5–6):219–226. https://doi.org/10.1016/j.drudis.2007.12.002
Carpenter KA et al (2018) Deep learning and virtual drug screening. Future Med Chem 10(21):2557–2567. https://doi.org/10.4155/fmc-2018-0314
Melville JL, Burke EK, Hirst JD (2009) Machine learning in virtual screening. Comb Chem High Throughput Screen 12(4):332–343. https://doi.org/10.2174/138620709788167980
Pereira JC, Caffarena ER, dos Santos CN (2016) Boosting docking-based virtual screening with deep learning. J Chem Inf Model 56(12):2495–2506. https://doi.org/10.1021/acs.jcim.6b00355
Ballester PJ, Mitchell JB (2010) A machine learning approach to predicting protein–ligand binding affinity with applications to molecular docking. Bioinformatics 26(9):1169–1175. https://doi.org/10.1093/bioinformatics/btq112
Zilian D, Sotriffer CA (2013) SFCscore(RF): a random forest-based scoring function for improved affinity prediction of protein–ligand complexes. J Chem Inf Model 53(8):1923–1933. https://doi.org/10.1021/ci400120b
Liu Q, Kwoh CK, Li J (2013) Binding affinity prediction for protein–ligand complexes based on β contacts and B factor. J Chem Inf Model 53(11):3076–3085. https://doi.org/10.1021/ci400450h
Li H et al (2014) istar: a web platform for large-scale protein–ligand docking. PLoS ONE 9(1):e85678. https://doi.org/10.1371/journal.pone.0085678
Li GB et al (2013) ID-Score: a new empirical scoring function based on a comprehensive set of descriptors related to protein–ligand interactions. J Chem Inf Model 53(3):592–600. https://doi.org/10.1021/ci300493w
Ballester PJ (2012) Machine learning scoring functions based on random forest and support vector regression. In: Proceedings of the 6th international conference on pattern recognition in bioinformatics. Springer, Berlin
Durrant JD, McCammon JA (2010) NNScore: a neural-network-based scoring function for the characterization of protein–ligand complexes. J Chem Inf Model 50(10):1865–1871. https://doi.org/10.1021/ci100244v
Ouyang X, Handoko SD, Kwoh CK (2011) CScore: a simple yet effective scoring function for protein–ligand binding affinity prediction using modified CMAC learning architecture. J Bioinform Comput Biol 9(Suppl 1):1–14. https://doi.org/10.1142/s021972001100577x
Cang Z, Wei G-W (2017) TopologyNet: topology based deep convolutional and multi-task neural networks for biomolecular property predictions. PLOS Comput Biol 13(7):e1005690. https://doi.org/10.1371/journal.pcbi.1005690
Ragoza M et al (2017) Protein–ligand scoring with convolutional neural networks. J Chem Inf Model 57(4):942–957. https://doi.org/10.1021/acs.jcim.6b00740
Stepniewska-Dziubinska MM, Zielenkiewicz P, Siedlecki P (2018) Development and evaluation of a deep learning model for protein–ligand binding affinity prediction. Bioinformatics 34(21):3666–3674. https://doi.org/10.1093/bioinformatics/bty374
Ashtawy HM, Mahapatra NR (2015) BgN-Score and BsN-Score: Bagging and boosting based ensemble neural networks scoring functions for accurate binding affinity prediction of protein–ligand complexes. BMC Bioinformatics 16(4):S8. https://doi.org/10.1186/1471-2105-16-S4-S8
Ain QU et al (2015) Machine-learning scoring functions to improve structure-based binding affinity prediction and virtual screening. Wiley Interdiscip Rev Comput Mol Sci 5(6):405–424. https://doi.org/10.1002/wcms.1225
Yang X et al (2019) Concepts of artificial intelligence for computer-assisted drug discovery. Chem Rev 119(18):10520–10594. https://doi.org/10.1021/acs.chemrev.8b00728
Wang D et al (2019) Improving the virtual screening ability of target-specific scoring functions using deep learning methods. Front Pharmacol 10:924. https://doi.org/10.3389/fphar.2019.00924
Li L et al (2011) Target-specific support vector machine scoring in structure-based virtual screening: computational validation, in vitro testing in kinases, and effects on lung cancer cell proliferation. J Chem Inf Model 51(4):755–759. https://doi.org/10.1021/ci100490w
Sullivan PF (2012) Puzzling over schizophrenia: schizophrenia as a pathway disease. Nat Med 18(2):210–211. https://doi.org/10.1038/nm.2670
Kottaram A et al (2019) Brain network dynamics in schizophrenia: reduced dynamism of the default mode network. Hum Brain Mapp 40(7):2212–2228. https://doi.org/10.1002/hbm.24519
Ermakov EA et al (2021) Oxidative stress-related mechanisms in schizophrenia pathogenesis and new treatment perspectives. Oxid Med Cell Longev 2021:8881770. https://doi.org/10.1155/2021/8881770
MacKay M-AB et al (2018) Multidimensional connectomics and treatment-resistant schizophrenia: linking phenotypic circuits to targeted therapeutics. Front Psychiatry 9:537. https://doi.org/10.3389/fpsyt.2018.00537
Perkovic MN et al (2017) Theranostic biomarkers for schizophrenia. Int J Mol Sci 18(4):733. https://doi.org/10.3390/ijms18040733
Saha S et al (2005) A systematic review of the prevalence of schizophrenia. PLoS Med 2(5):e141. https://doi.org/10.1371/journal.pmed.0020141
World Health Organization (2008) The global burden of disease: 2004 update. World Health Organization, Geneva. https://apps.who.int/iris/handle/10665/43942
Hyman SE (2012) Revolution stalled. Sci Transl Med 4(155):155cm11. https://doi.org/10.1126/scitranslmed.3003142
Yang QX et al (2019) Identification of the gene signature reflecting schizophrenia’s etiology by constructing artificial intelligence-based method of enhanced reproducibility. CNS Neurosci Ther 25(9):1054–1063. https://doi.org/10.1111/cns.13196
Zhao K, So HC (2019) Drug repositioning for schizophrenia and depression/anxiety disorders: a machine learning approach leveraging expression data. IEEE J Biomed Health Informatics 23(3):1304–1315. https://doi.org/10.1109/JBHI.2018.2856535
Chakravarty MM (2019) Guest editorial: special issue on machine learning in schizophrenia. Schizophr Res 214:1–2. https://doi.org/10.1016/j.schres.2019.10.044
Goedert M, Spillantini MG (2006) A century of Alzheimer’s disease. Science 314(5800):777–781. https://doi.org/10.1126/science.1132814
2021 Alzheimer’s disease facts and figures. Alzheimers Dement 17(3):327–406. https://doi.org/10.1002/alz.12328
Misra S, Medhi B (2013) Drug development status for Alzheimer’s disease: present scenario. Neurol Sci 34(6):831–839. https://doi.org/10.1007/s10072-013-1316-x
Cummings JL, Morstorf T, Zhong K (2014) Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimer’s Res Therapy 6(4):37. https://doi.org/10.1186/alzrt269
Louros N et al (2020) Structure-based machine-guided mapping of amyloid sequence space reveals uncharted sequence clusters with higher solubilities. Nat Commun 11(1):3314. https://doi.org/10.1038/s41467-020-17207-3
Sügis E et al (2019) HENA, heterogeneous network-based data set for Alzheimer’s disease. Sci Data 6(1):151. https://doi.org/10.1038/s41597-019-0152-0
Hung T-C et al (2014) In silico investigation of traditional Chinese medicine compounds to inhibit human histone deacetylase 2 for patients with Alzheimer’s disease. BioMed Res Int 2014:769867. https://doi.org/10.1155/2014/769867
Lee J et al (2019) Development of predictive models for identifying potential S100A9 inhibitors based on machine learning methods. Front Chem 7:779. https://doi.org/10.3389/fchem.2019.00779
Cavas L et al (2019) Neural network modeling of AChE inhibition by new carbazole-bearing oxazolones. Interdiscip Sci 11(1):95–107. https://doi.org/10.1007/s12539-017-0245-4
Jamal S, Grover A, Grover S (2019) Machine learning from molecular dynamics trajectories to predict caspase-8 inhibitors against Alzheimer’s disease. Front Pharmacol 10:780. https://doi.org/10.3389/fphar.2019.00780
Zhang X-M et al (2021) Graph neural networks and their current applications in bioinformatics. Front Genet. https://doi.org/10.3389/fgene.2021.690049
Miyazaki Y et al (2020) Comprehensive exploration of target-specific ligands using a graph convolution neural network. Mol Inform 39(1–2):e1900095. https://doi.org/10.1002/minf.201900095
Kleandrova VV, Speck-Planche A (2020) PTML modeling for Alzheimer’s disease: design and prediction of virtual multi-target inhibitors of GSK3B, HDAC1, and HDAC6. Curr Top Med Chem 20(19):1661–1676. https://doi.org/10.2174/1568026620666200607190951
Gupta R, Ambasta RK, Kumar P (2020) Identification of novel class I and class IIb histone deacetylase inhibitor for Alzheimer’s disease therapeutics. Life Sci 256:117912. https://doi.org/10.1016/j.lfs.2020.117912
Fang J et al (2017) AlzhCPI: A knowledge base for predicting chemical–protein interactions towards Alzheimer’s disease. PLoS ONE 12(5):e0178347. https://doi.org/10.1371/journal.pone.0178347
Fang J et al (2015) Discovery of multitarget-directed ligands against Alzheimer’s disease through systematic prediction of chemical–protein interactions. J Chem Inf Model 55(1):149–164. https://doi.org/10.1021/ci500574n
Pang XC et al (2018) Network pharmacology-based analysis of Chinese herbal Naodesheng formula for application to Alzheimer’s disease. Chin J Nat Med 16(1):53–62. https://doi.org/10.1016/s1875-5364(18)30029-3
Grisoni F et al (2019) Design of natural-product-inspired multitarget ligands by machine learning. ChemMedChem 14(12):1129–1134. https://doi.org/10.1002/cmdc.201900097
Thompson CA (2001) FDA approves galantamine for Alzheimer’s disease. Am J Health Syst Pharm 58(8):649. https://doi.org/10.1093/ajhp/58.8.649a
Jamal S et al (2016) Integrating network, sequence and functional features using machine learning approaches towards identification of novel Alzheimer genes. BMC Genomics 17(1):807. https://doi.org/10.1186/s12864-016-3108-1
Exscientia announces second molecule created using AI from Sumitomo Dainippon Pharma collaboration to enter Phase 1 clinical trial. Cited 16 Sept 2021. https://www.exscientia.ai/news-insights/exscientia-second-ai-molecule-from-collaboration-in-phase1
Oh M, Ahn J, Yoon Y (2014) A network-based classification model for deriving novel drug-disease associations and assessing their molecular actions. PLoS ONE 9(10):e111668. https://doi.org/10.1371/journal.pone.0111668
Dorsey ER et al (2018) The emerging evidence of the Parkinson pandemic. J Parkinsons Dis 8(s1):S3–S8. https://doi.org/10.3233/jpd-181474
Reeve A, Simcox E, Turnbull D (2014) Ageing and Parkinson’s disease: why is advancing age the biggest risk factor? Ageing Res Rev 14(100):19–30. https://doi.org/10.1016/j.arr.2014.01.004
Cerri S, Mus L, Blandini F (2019) Parkinson’s disease in women and men: what’s the difference? J Parkinsons Dis 9(3):501–515. https://doi.org/10.3233/jpd-191683
Pinto M et al (2019) Boosting drug discovery for Parkinson’s: enhancement of the delivery of a monoamine oxidase-b inhibitor by brain-targeted PEGylated polycaprolactone-based nanoparticles. Pharmaceutics. https://doi.org/10.3390/pharmaceutics11070331
Taylor JP, Hardy J, Fischbeck KH (2002) Toxic proteins in neurodegenerative disease. Science 296(5575):1991–1995. https://doi.org/10.1126/science.1067122
Maclagan LC et al (2020) Identifying drugs with disease-modifying potential in Parkinson’s disease using artificial intelligence and pharmacoepidemiology. Pharmacoepidemiol Drug Saf 29(8):864–872. https://doi.org/10.1002/pds.5015
Peng J, Guan J, Shang X (2019) Predicting Parkinson’s disease genes based on Node2vec and Autoencoder. Front Genet 10:226. https://doi.org/10.3389/fgene.2019.00226
Matarazzo M et al (2019) Remote monitoring of treatment response in Parkinson’s disease: the habit of typing on a computer. Mov Disord 34(10):1488–1495. https://doi.org/10.1002/mds.27772
Potashkin JA et al (2012) Biosignatures for Parkinson’s disease and atypical parkinsonian disorders patients. PLoS ONE 7(8):e43595. https://doi.org/10.1371/journal.pone.0043595
Váradi C et al (2019) Serum N-glycosylation in Parkinson’s disease: a novel approach for potential alterations. Molecules. https://doi.org/10.3390/molecules24122220
Verge genomics: employing AI to improve drug discovery (2018). Pharma Technology Focus, New York
Burik A (2018) AI is being put to work to treat Parkinson’s disease in the UK. Labiotech.eu. https://www.labiotech.eu/trends-news/benevolent-ai-parkinsons-disease/
Yele V, Azam MA, Jupudi S (2020) Ligand-based pharmacophore modelling, in silico virtual screening, molecular docking and molecular dynamic simulation study to identify novel Francisella tularensis ParE inhibitors. Chem Pap 74(12):4567–4580. https://doi.org/10.1007/s11696-020-01274-3
Ferraz WR et al (2020) Ligand and structure-based virtual screening applied to the SARS-CoV-2 main protease: an in silico repurposing study. Future Med Chem 12(20):1815–1828. https://doi.org/10.4155/fmc-2020-0165
Liu C et al (2020) Pharmacophore-based virtual screening toward the discovery of novel anti-echinococcal compounds. Front Cell Infect Microbiol. https://doi.org/10.3389/fcimb.2020.00118
Cheng T et al (2012) Structure-based virtual screening for drug discovery: a problem-centric review. AAPS J 14(1):133–141. https://doi.org/10.1208/s12248-012-9322-0
Maia EHB et al (2020) Structure-based virtual screening: from classical to artificial intelligence. Front Chem. https://doi.org/10.3389/fchem.2020.00343
Negi P, Prakash S, Patil VM (2021) Structure based drug design approach to identify potential SARS-CoV-2 polymerase inhibitors. Coronaviruses 2(4):507–515. https://doi.org/10.2174/2666796701999201113114545
Vázquez J et al (2020) Merging ligand-based and structure-based methods in drug discovery: an overview of combined virtual screening approaches. Molecules 25(20):4723
Wilson GL, Lill MA (2011) Integrating structure-based and ligand-based approaches for computational drug design. Future Med Chem 3(6):735–750. https://doi.org/10.4155/fmc.11.18
Sliwoski G et al (2014) Computational methods in drug discovery. Pharmacol Rev 66(1):334–395. https://doi.org/10.1124/pr.112.007336
Klopmand G (1990) In: Johnson MA, Maggiora GM (eds) Concepts and applications of molecular similarity. Wiley, New York, p 393
Plewczynski D, Spieser SA, Koch U (2009) Performance of machine learning methods for ligand-based virtual screening. Comb Chem High Throughput Screen 12(4):358–368. https://doi.org/10.2174/138620709788167962
Jayaraj PB, Jain S (2019) Ligand based virtual screening using SVM on GPU. Comput Biol Chem 83:107143. https://doi.org/10.1016/j.compbiolchem.2019.107143
Ma XH et al (2009) Comparative analysis of machine learning methods in ligand-based virtual screening of large compound libraries. Comb Chem High Throughput Screen 12(4):344–357. https://doi.org/10.2174/138620709788167944
Fukunishi Y (2009) Structure-based drug screening and ligand-based drug screening with machine learning. Comb Chem High Throughput Screen 12(4):397–408. https://doi.org/10.2174/138620709788167890
Quintus F et al (2009) Ligand scaffold hopping combining 3D maximal substructure search and molecular similarity. BMC Bioinformatics 10:245. https://doi.org/10.1186/1471-2105-10-245
Jain AN (2004) Ligand-based structural hypotheses for virtual screening. J Med Chem 47(4):947–961. https://doi.org/10.1021/jm030520f
Briard JG et al (2016) QSAR accelerated discovery of potent ice recrystallization inhibitors. Sci Rep 6:26403. https://doi.org/10.1038/srep26403
Kumar R et al (2015) An in silico platform for predicting, screening and designing of antihypertensive peptides. Sci Rep 5:12512. https://doi.org/10.1038/srep12512
Wang T et al (2015) Quantitative structure–activity relationship: promising advances in drug discovery platforms. Expert Opin Drug Discov 10(12):1283–1300. https://doi.org/10.1517/17460441.2015.1083006
Geanes AR et al (2016) Ligand-based virtual screen for the discovery of novel M5 inhibitor chemotypes. Bioorg Med Chem Lett 26(18):4487–4491. https://doi.org/10.1016/j.bmcl.2016.07.071
Myint KZ et al (2012) Molecular fingerprint-based artificial neural networks QSAR for ligand biological activity predictions. Mol Pharm 9(10):2912–2923. https://doi.org/10.1021/mp300237z
Patra JC, Chua BH (2011) Artificial neural network-based drug design for diabetes mellitus using flavonoids. J Comput Chem 32(4):555–567. https://doi.org/10.1002/jcc.21641
Hu L, Chen G, Chau RM (2006) A neural networks-based drug discovery approach and its application for designing aldose reductase inhibitors. J Mol Graph Model 24(4):244–253. https://doi.org/10.1016/j.jmgm.2005.09.002
Khatri N, Lather V, Madan AK (2014) Diverse classification models for anti-hepatitis C virus activity of thiourea derivatives. Chemom Intell Lab Syst. https://doi.org/10.1016/j.chemolab.2014.10.007
Torrent M et al (2011) Connecting peptide physicochemical and antimicrobial properties by a rational prediction model. PLoS ONE 6(2):e16968. https://doi.org/10.1371/journal.pone.0016968
Fjell CD et al (2009) Identification of novel antibacterial peptides by chemoinformatics and machine learning. J Med Chem 52(7):2006–2015. https://doi.org/10.1021/jm8015365
Sabet R et al (2012) Computer-aided design of novel antibacterial 3-hydroxypyridine-4-ones: application of QSAR methods based on the MOLMAP approach. J Comput Aided Mol Des 26(3):349–361. https://doi.org/10.1007/s10822-012-9561-2
Douali L, Villemin D, Cherqaoui D (2003) Neural networks: Accurate nonlinear QSAR model for HEPT derivatives. J Chem Inf Comput Sci 43(4):1200–1207. https://doi.org/10.1021/ci034047q
Murcia-Soler M et al (2004) Artificial neural networks and linear discriminant analysis: a valuable combination in the selection of new antibacterial compounds. J Chem Inf Comput Sci 44(3):1031–1041. https://doi.org/10.1021/ci030340e
AbdulHameed MD, Ippolito DL, Wallqvist A (2016) Predicting rat and human pregnane X receptor activators using Bayesian classification models. Chem Res Toxicol 29(10):1729–1740. https://doi.org/10.1021/acs.chemrestox.6b00227
Renault N et al (2013) Virtual screening of CB(2) receptor agonists from Bayesian network and high-throughput docking: structural insights into agonist-modulated GPCR features. Chem Biol Drug Des 81(4):442–454. https://doi.org/10.1111/cbdd.12095
Singh N et al (2012) QSAR classification model for antibacterial compounds and its use in virtual screening. J Chem Inf Model 52(10):2559–2569. https://doi.org/10.1021/ci300336v
Liu L-l et al (2014) Novel Bayesian classification models for predicting compounds blocking hERG potassium channels. Acta Pharmacol Sin 35(8):1093–1102. https://doi.org/10.1038/aps.2014.35
Vijayan RSK et al (2009) Combinatorial library enumeration and lead hopping using comparative interaction fingerprint analysis and classical 2D QSAR methods for seeking novel GABAA α3 modulators. J Chem Inf Model 49(11):2498–2511. https://doi.org/10.1021/ci900309s
Ekins S et al (2013) Bayesian models leveraging bioactivity and cytotoxicity information for drug discovery. Chem Biol 20(3):370–378. https://doi.org/10.1016/j.chembiol.2013.01.011
Prathipati P, Ma NL, Keller TH (2008) Global Bayesian models for the prioritization of antitubercular agents. J Chem Inf Model 48(12):2362–2370. https://doi.org/10.1021/ci800143n
Bender A, Mussa HY, Glen RC (2005) Screening for dihydrofolate reductase inhibitors using MOLPRINT 2D, a fast fragment-based method employing the naïve Bayesian classifier: limitations of the descriptor and the importance of balanced chemistry in training and test sets. J Biomol Screen 10(7):658–666. https://doi.org/10.1177/1087057105281048
Xia X et al (2004) Classification of kinase inhibitors using a Bayesian model. J Med Chem 47(18):4463–4470. https://doi.org/10.1021/jm0303195
Chen JJ, Visco DP (2017) Identifying novel factor XIIa inhibitors with PCA-GA-SVM developed vHTS models. Eur J Med Chem 140:31–41
Chen JJ, Visco DP (2017) Developing an in silico pipeline for faster drug candidate discovery: virtual high throughput screening with the Signature molecular descriptor using support vector machine models. Chem Engg Sci 159:31–42
Fang X, Bagui S, Bagui S (2017) Improving virtual screening predictive accuracy of Human kallikrein 5 inhibitors using machine learning models. Comput Biol Chem 69:110–119. https://doi.org/10.1016/j.compbiolchem.2017.05.007
Zakharov AV et al (2016) QSAR Modeling and prediction of drug–drug interactions. Mol Pharm 13(2):545–556. https://doi.org/10.1021/acs.molpharmaceut.5b00762
Svetnik V et al (2003) Random forest: a classification and regression tool for compound classification and QSAR modeling. J Chem Inf Comput Sci 43(6):1947–1958. https://doi.org/10.1021/ci034160g
Ma J et al (2015) Deep neural nets as a method for quantitative structure–activity relationships. J Chem Inf Model 55(2):263–274. https://doi.org/10.1021/ci500747n
Martin EJ et al (2017) Profile-QSAR 2.0: kinase virtual screening accuracy comparable to four-concentration IC(50)s for realistically novel compounds. J Chem Inf Model 57(8):2077–2088. https://doi.org/10.1021/acs.jcim.7b00166
Shamsara J (2019) A random forest model to predict the activity of a large set of soluble epoxide hydrolase inhibitors solely based on a set of simple fragmental descriptors. Comb Chem High Throughput Screen 22(8):555–569. https://doi.org/10.2174/1386207322666191016110232
Simeon S, Jongkon N (2019) Construction of quantitative structure activity relationship (QSAR) models to predict potency of structurally diversed Janus kinase 2 inhibitors. Molecules. https://doi.org/10.3390/molecules24234393
Marchese Robinson RL et al (2017) Comparison of the predictive performance and interpretability of random forest and linear models on benchmark data sets. J Chem Inf Model 57(8):1773–1792. https://doi.org/10.1021/acs.jcim.6b00753
Speck-Planche A, Kleandrova VV, Cordeiro MN (2013) New insights toward the discovery of antibacterial agents: multi-tasking QSBER model for the simultaneous prediction of anti-tuberculosis activity and toxicological profiles of drugs. Eur J Pharm Sci 48(4–5):812–818. https://doi.org/10.1016/j.ejps.2013.01.011
Speck-Planche A, Cordeiro MN (2013) Simultaneous modeling of antimycobacterial activities and ADMET profiles: a chemoinformatic approach to medicinal chemistry. Curr Top Med Chem 13(14):1656–1665. https://doi.org/10.2174/15680266113139990116
Speck-Planche A, Cordeiro MN (2014) Simultaneous virtual prediction of anti-Escherichia coli activities and ADMET profiles: a chemoinformatic complementary approach for high-throughput screening. ACS Comb Sci 16(2):78–84. https://doi.org/10.1021/co400115s
Kleandrova VV et al (2016) Enabling the discovery and virtual screening of potent and safe antimicrobial peptides. Simultaneous prediction of antibacterial activity and cytotoxicity. ACS Comb Sci 18(8):490–498. https://doi.org/10.1021/acscombsci.6b00063
Speck-Planche A, Dias Soeiro Cordeiro MN (2017) Speeding up early drug discovery in antiviral research: a fragment-based in silico approach for the design of virtual anti-hepatitis C leads. ACS Comb Sci 19(8):501–512. https://doi.org/10.1021/acscombsci.7b00039
Viña D et al (2009) Alignment-free prediction of a drug–target complex network based on parameters of drug connectivity and protein sequence of receptors. Mol Pharm 6(3):825–835. https://doi.org/10.1021/mp800102c
Speck-Planche A et al (2012) Chemoinformatics in multi-target drug discovery for anti-cancer therapy: in silico design of potent and versatile anti-brain tumor agents. Anticancer Agents Med Chem 12(6):678–685. https://doi.org/10.2174/187152012800617722
Speck-Planche A, Cordeiro M (2017) Fragment-based in silico modeling of multi-target inhibitors against breast cancer-related proteins. Mol Divers 21(3):511–523. https://doi.org/10.1007/s11030-017-9731-1
Dahl GE et al (2021) Multi-task neural networks for QSAR predictions. arXiv:1406.1231
Zakharov AV et al (2019) Novel consensus architecture to improve performance of large-scale multitask deep learning QSAR models. J Chem Inf Model 59(11):4613–4624. https://doi.org/10.1021/acs.jcim.9b00526
Kwon S et al (2019) Comprehensive ensemble in QSAR prediction for drug discovery. BMC Bioinformatics 20(1):521. https://doi.org/10.1186/s12859-019-3135-4
Liu SH et al (2018) Bayesian varying coefficient kernel machine regression to assess neurodevelopmental trajectories associated with exposure to complex mixtures. Stat Med 37(30):4680–4694. https://doi.org/10.1002/sim.7947
Maric NP et al (2016) Improving current treatments for schizophrenia. Drug Dev Res 77(7):357–367. https://doi.org/10.1002/ddr.21337
Marunnan SM et al (2017) Development of MLR and SVM aided QSAR models to identify common SAR of GABA uptake herbal inhibitors used in the treatment of schizophrenia. Curr Neuropharmacol 15(8):1085–1092. https://doi.org/10.2174/1567201814666161205131745
Hsu KC, Wang FS (2017) Model-based optimization approaches for precision medicine: a case study in presynaptic dopamine overactivity. PLoS ONE 12(6):e0179575. https://doi.org/10.1371/journal.pone.0179575
Luo M et al (2014) Application of quantitative structure-activity relationship models of 5-HT1A receptor binding to virtual screening identifies novel and potent 5-HT1A ligands. J Chem Inf Model 54(2):634–647. https://doi.org/10.1021/ci400460q
Luo M, Reid TE, Wang XS (2015) Discovery of natural product-derived 5-HT1A receptor binders by cheminfomatics modeling of known binders, high throughput screening and experimental validation. Comb Chem High Throughput Screen 18(7):685–692. https://doi.org/10.2174/1386207318666150703113948
Tan X et al (2020) Automated design and optimization of multitarget schizophrenia drug candidates by deep learning. Eur J Med Chem 204:112572. https://doi.org/10.1016/j.ejmech.2020.112572
Jebapriya S et al (2019) Support vector machine for classification of autism spectrum disorder based on abnormal structure of corpus callosum. Int J Adv Comput Sci Appl. https://doi.org/10.14569/IJACSA.2019.0100965
Gabrielsen M et al (2014) Identification of novel serotonin transporter compounds by virtual screening. J Chem Inf Model 54(3):933–943. https://doi.org/10.1021/ci400742s
Löber S et al (2011) Recent advances in the search for D3- and D4-selective drugs: probes, models and candidates. Trends Pharmacol Sci 32(3):148–157. https://doi.org/10.1016/j.tips.2010.12.003
Sibley DR, Monsma FJ Jr (1992) Molecular biology of dopamine receptors. Trends Pharmacol Sci 13(2):61–69. https://doi.org/10.1016/0165-6147(92)90025-2
Simpson MM et al (1999) Dopamine D4/D2 receptor selectivity is determined by A divergent aromatic microdomain contained within the second, third, and seventh membrane-spanning segments. Mol Pharmacol 56(6):1116–1126. https://doi.org/10.1124/mol.56.6.1116
Wang Q et al (2010) Subtype selectivity of dopamine receptor ligands: insights from structure and ligand-based methods. J Chem Inf Model 50(11):1970–1985. https://doi.org/10.1021/ci1002747
López L et al (2010) Synthesis, 3D-QSAR, and structural modeling of benzolactam derivatives with binding affinity for the D2 and D3 receptors. ChemMedChem 5(8):1300–1317. https://doi.org/10.1002/cmdc.201000101
Cho DI, Zheng M, Kim KM (2010) Current perspectives on the selective regulation of dopamine D2 and D3 receptors. Arch Pharm Res 33(10):1521–1538. https://doi.org/10.1007/s12272-010-1005-8
Carro L et al (2009) Synthesis and binding affinity of potential atypical antipsychotics with the tetrahydroquinazolinone motif. Bioorg Med Chem Lett 19(21):6059–6062. https://doi.org/10.1016/j.bmcl.2009.09.041
Huber D, Hübner H, Gmeiner P (2009) 1,1’-Disubstituted ferrocenes as molecular hinges in mono- and bivalent dopamine receptor ligands. J Med Chem 52(21):6860–6870. https://doi.org/10.1021/jm901120h
Han LY et al (2008) A support vector machines approach for virtual screening of active compounds of single and multiple mechanisms from large libraries at an improved hit-rate and enrichment factor. J Mol Graph Model 26(8):1276–1286. https://doi.org/10.1016/j.jmgm.2007.12.002
Li H et al (2007) Machine learning approaches for predicting compounds that interact with therapeutic and ADMET related proteins. J Pharm Sci 96(11):2838–2860. https://doi.org/10.1002/jps.20985
Mahe P, Vert J-P (2009) Virtual screening with support vector machines and structure kernels. Comb Chem High Throughput Screen 12(4):409–423. https://doi.org/10.2174/138620709788167926
Cha MY et al (2003) QSAR studies on piperazinylalkylisoxazole analogues selectively acting on dopamine D3 receptor by HQSAR and CoMFA. Bioorg Med Chem Lett 11:1293–1298. https://doi.org/10.1016/s0968-0896(02)00617-x
Audouze K, Nielsen E, Peters D (2004) New series of morpholine and 1,4-oxazepane derivatives as dopamine D4 receptor ligands: synthesis and 3D-QSAR model. J Med Chem 47(12):3089–3104. https://doi.org/10.1021/jm031111m
Clark R, Abrahamian E (2008) Using a staged multi-objective optimization approach to find selective pharmacophore models. J Comp Aided Mol Design 23:765–771. https://doi.org/10.1007/s10822-008-9227-2
Salama I et al (2007) Structure–selectivity investigations of D2-like receptor ligands by CoMFA and CoMSIA guiding the discovery of D3 selective PET radioligands. J Med Chem 50(3):489–500. https://doi.org/10.1021/jm0611152
Zhang J et al (2012) A two-step target binding and selectivity support vector machines approach for virtual screening of dopamine receptor subtype-selective ligands. PLoS ONE 7(6):e39076. https://doi.org/10.1371/journal.pone.0039076
Tsoumakas GK, Vlahavas I (2010) Mining multi-label data. In: Maimon O, Rokach L (eds) Data mining and knowledge discovery handbook. Springer, New York, pp 667–685
Ma XH et al (2010) Virtual screening of selective multitarget kinase inhibitors by combinatorial support vector machines. Mol Pharm 7(5):1545–1560. https://doi.org/10.1021/mp100179t
Tsoumakas G, Katakis I (2007) Multi-label classification: an overview. Int J Data Warehousing Min (IJDWM) 3(3):1–13
Schietgat L et al (2010) Predicting gene function using hierarchical multi-label decision tree ensembles. BMC Bioinformatics 11(1):2. https://doi.org/10.1186/1471-2105-11-2
Boeckler F, Gmeiner P (2006) The structural evolution of dopamine D3 receptor ligands: structure–activity relationships and selected neuropharmacological aspects. Pharmacol Ther 112(1):281–333. https://doi.org/10.1016/j.pharmthera.2006.04.007
Zhang J et al (2009) Dopamine D1 receptor ligands: where are we now and where are we going. Med Res Rev 29(2):272–294. https://doi.org/10.1002/med.20130
Herm L et al (2009) N-Substituted-2-alkyl- and 2-arylnorapomorphines: novel, highly active D-2 agonists. Bioorg Med Chem 17:4756–4762. https://doi.org/10.1016/j.bmc.2009.04.047
Gueiffier C, Gueiffier A (2006) Recent progress in medicinal chemistry of D-4 Agonists. Curr Med Chem 13:2981–2993. https://doi.org/10.2174/092986706778521841
Overington J (2009) ChEMBL. An interview with John Overington, team leader, chemogenomics at the European Bioinformatics Institute Outstation of the European Molecular Biology Laboratory (EMBL-EBI). Interview by Wendy A. Warr. J Comput Aided Mol Des 23(4):195–198. https://doi.org/10.1007/s10822-009-9260-9
Durrant JD, McCammon JA (2010) Computer-aided drug-discovery techniques that account for receptor flexibility. Curr Opin Pharmacol 10(6):770–774. https://doi.org/10.1016/j.coph.2010.09.001
Sun H (2008) Pharmacophore-based virtual screening. Curr Med Chem 15(10):1018–1024. https://doi.org/10.2174/092986708784049630
Sprous DG et al (2010) QSAR in the pharmaceutical research setting: QSAR models for broad, large problems. Curr Top Med Chem 10(6):619–637. https://doi.org/10.2174/156802610791111506
Willett P (2011) Similarity searching using 2D structural fingerprints. Methods Mol Biol 672:133–158. https://doi.org/10.1007/978-1-60761-839-3_5
Talevi A et al (2009) Combined virtual screening strategies. Curr Comput Aided Drug Des 5(1):23–37. https://doi.org/10.2174/157340909787580854
Fulp J et al (2018) Structural insights of benzenesulfonamide analogues as NLRP3 inflammasome inhibitors: design, synthesis, and biological characterization. J Med Chem 61(12):5412–5423. https://doi.org/10.1021/acs.jmedchem.8b00733
Chen Z-D et al (2020) A novel artificial intelligence protocol to investigate potential leads for Parkinson’s disease. RSC Adv 10(39):22939–22958. https://doi.org/10.1039/D0RA04028B
Nedaie A, Najafi AA (2018) Support vector machine with Dirichlet feature mapping. Neural Netw 98:87–101. https://doi.org/10.1016/j.neunet.2017.11.006
Speybroeck N (2012) Classification and regression trees. Int J Public Health 57(1):243–246. https://doi.org/10.1007/s00038-011-0315-z
Carpenter KA, Huang X (2018) Machine learning-based virtual screening and its applications to Alzheimer’s drug discovery: a review. Curr Pharm Des 24(28):3347–3358. https://doi.org/10.2174/1381612824666180607124038
Kumar A, Srivastava G, Sharma A (2017) A physicochemical descriptor based method for effective and rapid screening of dual inhibitors against BACE-1 and GSK-3β as targets for Alzheimer’s disease. Comput Biol Chem 71:1–9. https://doi.org/10.1016/j.compbiolchem.2017.09.001
Chen Y et al (2015) Discovery of new acetylcholinesterase inhibitors with small core structures through shape-based virtual screening. Bioorg Med Chem Lett 25(17):3442–3446. https://doi.org/10.1016/j.bmcl.2015.07.026
Masand N et al (2015) Heterocyclic secretase inhibitors for the treatment of Alzheimer’s disease: an overview. Cent Nerv Syst Agents Med Chem 17(1):3–25. https://doi.org/10.2174/1570159X13666151029105752
Gupta SP, Patil VM (2020) Recent studies on design and development of drugs against Alzheimer’s disease (AD) based on inhibition of BACE-1 and other AD-causative agents. Curr Top Med Chem 20(13):1195–1213. https://doi.org/10.2174/1568026620666200416091623
Bhardwaj M et al (2019) Anti-acetylcholinesterase derivatives: a privileged structural framework in drug discovery to treat Alzheimer’s disease. Curr Enzyme Inhibition 15:8–21. https://doi.org/10.2174/1573407215666190111150241
Ambure P et al (2019) Identifying natural compounds as multi-target-directed ligands against Alzheimer’s disease: an in silico approach. J Biomol Struct Dyn 37(5):1282–1306. https://doi.org/10.1080/07391102.2018.1456975
Funding
None.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest, financial or otherwise.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gautam, V., Gaurav, A., Masand, N. et al. Artificial intelligence and machine-learning approaches in structure and ligand-based discovery of drugs affecting central nervous system. Mol Divers 27, 959–985 (2023). https://doi.org/10.1007/s11030-022-10489-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10489-3