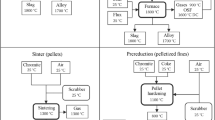
Work is devoted to solution of the problem of processing dross obtained as a result of hot galvanizing of products, in particular, waste-free utilization of its oxidized component by chlorination firing using cheap chlorine-containing CaCl2 and NH4Cl reagents. Results of chlorination roasting of the dross oxidized component together with CaCl2 and NH4Cl at a 1000°C are presented. The effect of CaCl2 and NH4Cl consumption on lead and iron sublimation from the dross oxidized component in the form of chlorides is studied. It is found that use of CaCl2 with a consumption equal to 6% of the weight of the oxidized component provides thorough purification of the starting material from Pb, Cu, Ni, Cd. Maximum reduction of the iron content is not possible. It is shown that use of NH4Cl with a consumption of 15% of the weight of the oxidized component provides thorough removal of impurity metals and metallic zinc from the source material. It is found that with a firing temperature of 1000°C thorough sublimation of impurities is achieved, which ensures production of pure zinc oxide, suitable for use as a mineral additive in animal and bird feed. The content of metal impurities in the resulting zinc oxide is, wt.%: Pb 0.02, Fe 0.08, Ni 0.02, Cu 0.008, Cd 0.001. Optimum firing parameters are established: T = 1000°C, duration 60 min, air flow 0.1 liter/min, CaCl2 consumption 6%, and NH4Cl 15% of the weight of the oxidized component.





Similar content being viewed by others
References
Ya. M. Kolotyrkin, Metal and Corrosion [in Russian], Metallurgiya, Moscow (1985).
G. G. Ulig and R. O. Revi, Corrosion and Combatting it: Introduction to Corrosion Science and Technology [in Russian], Khimiya, Leningrad (1989).
V. M. Konstantinov, D. V. Gegenya, and M. I. Bogdanchik, “Review of the market for zinc and zinc waste,” Lit. Protsessy, No. 3, 244-250 (2014).
G. Stubbe, C. Hillmann, and C. Wolf, “Zinc and iron recovery from filter dust by melt bath injection into an induction furnace,” Erzmetall, 69 , No. 3, 5–12 (2016).
Z. Takácová, B. Hluchánová, and J. Trpcevská, “Leaching of zinc from zinc ash originating from hot-dip galvanizing,” Metall, 64, No. 12, 517–519 (2010).
P. P. Chernov, A. N. Karyshev, Yu. I. Larin, et al., RF Patent 2188244, MPK С22В7/00. Method for Preparing Zinc from Zinc Dross, No. 2001109810/02; Claim 11.02.2001; Publ. 27.08.2002. Bull. No. 24.
R. A. Yudin, A. V. Vinogradov, S. V. Kovryakov, É. A. Sudakov, and A. N. Yanichev, RF Patent 2369650, МPК С22В7/00. Device and Method for Extracting Zinc from Zinc Ddross, No. 2008102795/02; Claim 10.10.2009; Publ. 10.10.2009, Bull. No. 28.
D. Saramak, D. Krawczykowski, and T. Gawenda, “Investigations of zinc recovery from metallurgical waste,” IOP Conf. Series: Mater. Sci. and Eng., 427, No. 1, 012017 (2018).
J. Trpcevska, et al., “Leaching of zinc ash with hydrochloric acid solutions,” Polish J. of Environmental Studies, 27, No. 4, 1785–1771 (2018).
S. Najiba, “Recovery of zinc from ash of galvanizing plant by hydrometallurgical route. Bangladesh University of Engineering and Technology,” in: The Thesis of Master of Science in Materials and Metallurgical Engineering Department (2009), pp. 1–92.
А. В. Таrаsоv, “Hot galvanizing waste treatment,” Stal’, No. 6, 57–58 (1989).
H. Wang, Y. Feng, H. Li, and J. Kang, “Simultaneous extraction of gold and zinc from refractory carbonaceous gold ore by chlorination roasting process,” Trans. Nonferrous Met. Soc. China, 30, No. 4, 1111 (2020).
X. Guo, B. Zhang, Q. Wang, Z. Li, and Q. Tian, “Recovery of zinc and lead from copper smelting slags by chlorination roasting,” JOM, 73, 1861–1870 (2021).
S. Bai, Y. Bi, Z. Ding, C. Li, and S. Wen, “Innovative methodology for the utilization of low-grade pyrite cinder containing heavy metals via hydrothermal alkali melting followed by chlorination roasting,” J. Alloys Compd., 840, 155722 (2020).
G. M. Koishina, E. E. Zholdasbay, M. B. Kurmanseitov, E. B. Tazhiev, and A. A. Argyn, “Study on the behavior of zinc and associated metal-impurities in the process of chlorinating roasting of dross,” Complex Use of Mineral Resources (KIMS), 3, 71–80 (2021).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, Vol. 66, No. 3, pp. 85–91, March, 2022. Russian DOI https://doi.org/10.52351/00260827_2022_03_85.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dosmukhamedov, N.K., Zholdasbai, E.E., Koishina, G.M. et al. Chlorination Roasting of Oxidized Component Obtained from Dross at a Temperature of 1000°C. Metallurgist 66, 335–342 (2022). https://doi.org/10.1007/s11015-022-01333-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-022-01333-y