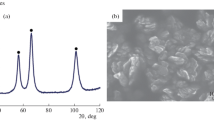
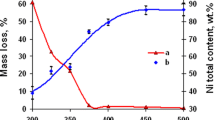
We study the kinetic characteristics and properties of the product of the process of synthesizing nickel nanopowders by the hydrogen reduction of Ni(OH)2 hydroxide compound under nonisothermal conditions. It is shown that the process of hydrogen reduction of the nanopowder of Ni(OH)2 hydroxide takes place within the temperature range 210–300°C with the maximal specific rate equal to 9.44·10−8 kg/sec recorded at a temperature of 285°C. The activation energy of the process of reduction of Ni(OH)2 nanopowder under nonisothermal conditions was estimated as ≈ 54 kJ/mole, which confirms the kinetic conditions of limiting of the process. It is shown that the elevation of temperature up to 285°C enables us to efficiently increase the rate of the total process of hydrogen reduction of Ni(OH)2 nanopowder (with guaranteeing high quality of the products of reduction). The obtained Ni nanoparticles mainly have round shapes and the sizes from ten to several tens of nanometers with a mean value of 50 nm.





Similar content being viewed by others
Change history
10 July 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11015-021-01179-w
References
B. Bhushan, Springer Handbook of Nanotechnology, 4th edn., Springer, Berlin (2017).
S. P. Bardakhanov, Yu. Ya. Gafner, et al., “Production of nickel nanopowders by means of evaporation of the initial coarse-dispersed substance in an electron accelerator,” Fiz. Tverd. Tela, 53, No. 4, 797–802 (2011).
I. V. Blinkov, A. O. Volkhonskii, D. S. Belov, V. I. Blinkov, R. L. Shatalov, and V. A. Andreev, “Properties of nanostructural ceramic-metallic TiN–Ni coatings obtained by the ion-plasma vacuum-arc method,” Izv. Vyssh. Uchebn. Zaved., Tsvet. Metallurg., No. 4, 51–58 (2014).
T. Ashokkumar, A. Rajadurai, Gouthama, and S. C. B. Gopinath, “A study of magnetic retardation of iron-nickel nanopowder and sintered specimen by spark plasma,” J. Magn. Magn. Mater., 485, 280–285 (2019).
M. I. Alymov, N. M. Rubtsov, and B. S. Seplyarskii, “Synthesis of nickel nanopowders under dynamic conditions,” Nanotechnol. Russia, 13, No. 11, 557–560 (2018).
M. K. Kesarla, N. N. K. Reddy, and F. Ortiz-Chi, “Transformation of g-C3N4 into onion like carbon on nickel nanoparticles for ultrafast hydrogenation,” Mater. Chem. Phys., 240, Issue 122, 157–162 (2020).
V. M. Nguyen, G. Karunakaran, T. H. Nguyen, E. A. Kolesnikov, M. I. Alymov, V. V. Levina, and Y. V. Konyukhov, “Enhancement of structural and mechanical properties of Fe+0.5% C steel powder alloy via incorporation of Ni and Co nanoparticles,” Lett. Mater., 10, No. 2, 174–178 (2020).
E. N. Sidorova, E. L. Dzidziguri, and V. V. Levina, “Dispersed characteristics of nickel nanopowders,” Metally, No. 6, 78–82 (2008).
K. H. Kim, H. C. Park, S. D. Lee, W. J. Hwa, S. S. Hong, G. D. Lee, and S. S. Park, “Preparation of submicron nickel powders by microwave-assisted hydrothermal method,” Mater. Chem. Phys., 92, No. 1, 234 (2005).
S. Banic and A. Mahajan, “Size control synthesis of pure nickel nanoparticles and anodic-oxidation of Butan-1-ol in alkali,” Mater. Chem. Phys., 235 (121), 747–756 (2019).
G. Partizan, B. Z. Mansurov, et al., “Investigation of electroexplosive nickel nanopowders,” Zh. Tekh. Fiz., 86, No. 11, 86–90 (2016).
I. Dulina, T. Lobunets, L. Klochkov, and A. Ragulya, “Obtaining of Ni/NiO nanopowder from aqua solutions of Ni(CH3COO)2 ammonia complexes, ” Nanoscale Res. Lett., 10, 156 (2015).
Z. Wei, P. Yan, and W. Feng, “Microstructural characterization of Ni nanoparticles prepared by anodic arc plasma,” Mater. Charact., 57, 176–181 (2006).
K. Y. Jung, J. H. Lee, H. Y. Koo, Y. C. Kang, and S. B. Park, “Preparation of solid nickel nanoparticles by large-scale spray pyrolysis of Ni(NO3)2·6 H2O precursor: Effect of temperature and nickel acetate on the particle morphology,” Mater. Sci. Eng., Ser. B, 137, Issue 1-3, 10–19 (2007).
D. I. Ryzhonkov, Yu. V. Konyukhov, and V. M. Nguyen, “Kinetic regularities and mechanisms of hydrogen reduction of nanosized oxide materials in thin layers,” Nanotechnol. Russia, 12, No. 11-12, 620–626 (2017).
V. M. Nguyen, Y. V. Konyukhov, and D. I. Ryzhonkov, “Influence of a rotary electromagnetic field and mechanical stimulation on the production of cobalt nanopowder by reduction with hydrogen,” Steel Trans., 48, No. 2, 73–77 (2018).
Y. V. Konyukhov, V. M. Nguyen, and D. I. Ryzhonkov, “Kinetics of reduction of α-Fe2O3 nanopowder with hydrogen under power mechanical treatment in an electromagnetic field,” Inorg. Mater. Appl. Res., 10, No. 3, 706–712 (2019).
T. H. Nguyen, G. Karunakaran, Y. V. Konyukhov, N. V. Minh, D. Y. Karpenkov, and I. N. Burmistrov, “Impact of iron on the Fe–Co–Ni ternary nanocomposites structural and magnetic features obtained via chemical precipitation followed by reduction process for various magnetically coupled devices applications,” Nanomaterials, 11, No. 2, 341 (1–14) (2021).
Yu. V. Sharikov and Ts. F. Liu, “Mathematical modeling of the process of reduction of nickel monoxide in a rotating tubular furnace,” Metallurg, No. 7, 27–32 (2018).
N. A. Zyuban, I. L. Gonik, and N. A. Novitskii, “Influence of the multicomponent binder on the reduction of iron from oxides in scale-carbon briquettes,” Metallurg, No. 7, 38–41 (2014).
B. P. Kulikov, V. N. Baranov, A. I. Bezrukikh, V. B. Deev, and M. M. Motkov, “Production of aluminum-scandium alloying composition by the aluminum-thermal reduction of scandium fluoride extracted from Sc2O3,” Metallurg, No. 12, 70–74 (2017).
T. H. Nguyen, V. M. Nguyen, V. N. Danchuk, M. H. Nguyen, H. V. Nguyen, and X. V. Tang, “Kinetic characteristics of the process of synthesis of nickel nanopowder by the chemical metallurgy method,” Nanotechnol. Russia, 15, No. 2, 146–152 (2020).
T. Kh. Nguyen and N. V. Min’, “Kinetics of the process of production of iron nanopowder by the chemical-metallurgical method under isothermal conditions,” Chern. Met., No. 1, 72–77 (2021).
M. E. Brown, D. Dollimore, and A. K. Galweys, Reactions in the Solid State, Elsevier, Reading (1980).
H. Schmalzried, Chemical Kinetics of Solids, VCH, Weinheim (1995).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, Vol. 65, No. 2, pp. 69–73, February, 2020.
Rights and permissions
About this article
Cite this article
Nguyen, V.M., Nguyen, T.H. Investigation of the Process of Synthesizing Nanosized Nickel Powders by Hydrogen Reduction Under Nonisothermal Conditions. Metallurgist 65, 206–213 (2021). https://doi.org/10.1007/s11015-021-01149-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-021-01149-2