Summary
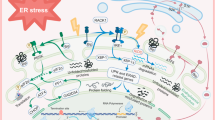
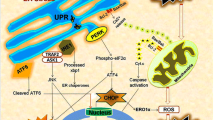
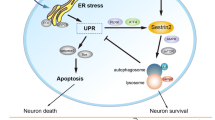
Traumatic brain injury (TBI), as a serious central nervous system disease, can result in severe neurological dysfunction or even disability and death of patients. The early and effective intervention of secondary brain injury can improve the prognosis of TBI. Endoplasmic reticulum (ER) stress is one of the main reasons to recover TBI. ER stress inhibition may be beneficial in treating TBI. Sestrin2 is a crucial regulator of ER stress, and its activation can significantly improve TBI. In this paper, we analyze the biological function of sestrin2, the latest findings on ER stress, and the relationship between ER stress and TBI. We elucidate the relationship of sestrin2 inhibiting ER stress via activating the AMP-activated protein kinase (AMPK)/mammalian target of rapamycin complex 1 (MTORC1) signaling. Finally, we elaborate on the possible role of sestrin2 in TBI and explain how its activation potentially improves TBI.



Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- TBI:
-
Traumatic brain injury
- ER:
-
Endoplasmic reticulum
- AMPK:
-
AMP-activated protein kinase
- mTORC1:
-
Mammalian target of rapamycin complex 1
- TSC:
-
Tuberous sclerosis complex
- PA26:
-
p53-activated gene 26
- ATF4:
-
Activating transcription factor 4
- Nrf2:
-
Nuclear factor erythroid-2-related factor 2
- WDR:
-
WD repeat domain 24
- GATOR1:
-
GTPase-activating protein activity toward Rags1
- RNF167:
-
RING finger protein 167
- STAMBPL1:
-
STAM-binding-protein-like 1
- UPR:
-
Unfolded protein response
- CHOP:
-
C/EBP homologous protein
- IRE1:
-
Inositol required kinase 1
- PERK:
-
Protein kinase R-like endoplasmic reticulum kinase
- mTOR:
-
Mechanistic target of rapamycin
- ULK1:
-
Unc-51 like autophagy activating kinase 1
- I/R:
-
Ischemia-reperfusion
- NF-κB:
-
Nuclear factor kappa B
- ROS:
-
Reactive oxygen species
- TON:
-
Traumatic optic neuropathy
- PDIA3:
-
Protein disulfide isomerase A3
- DNAJC3:
-
DNA J homology subfamily C member 3
- TBK1:
-
TANK-binding kinase 1
- PD:
-
Parkinson’s disease
- AD:
-
Alzheimer’s disease
References
Ambrosio S, Saccà CD, Amente S, Paladino S, Lania L, Majello B (2017) Lysine-specific demethylase LSD1 regulates autophagy in neuroblastoma through SESN2-dependent pathway. Oncogene 36(48):6701–6711. https://doi.org/10.1038/onc.2017.267
Budanov AV, Karin M (2008) p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 134(3):451–460. https://doi.org/10.1016/j.cell.2008.06.028
Byun JK, Choi YK, Kim JH, Jeong JY, Jeon HJ, Kim MK, Hwang I, Lee SY, Lee YM, Lee IK, Park KG (2017) A positive Feedback Loop between Sestrin2 and mTORC2 is required for the survival of glutamine-depleted Lung Cancer cells. Cell Rep 20(3):586–599. https://doi.org/10.1016/j.celrep.2017.06.066
Cangelosi AL, Puszynska AM, Roberts JM, Armani A, Nguyen TP, Spinelli JB, Kunchok T, Wang B, Chan SH, Lewis CA, Comb WC, Bell GW, Helman A, Sabatini DM (2022) Zonated leucine sensing by Sestrin-mTORC1 in the liver controls the response to dietary leucine. Science 377(6601):47–56. https://doi.org/10.1126/science.abi9547
Chantranupong L, Wolfson RL, Orozco JM, Saxton RA, Scaria SM, Bar-Peled L, Spooner E, Isasa M, Gygi SP, Sabatini DM (2014) The sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell Rep 9(1):1–8. https://doi.org/10.1016/j.celrep.2014.09.014
Chen YS, Chen SD, Wu CL, Huang SS, Yang DI (2014) Induction of sestrin2 as an endogenous protective mechanism against amyloid beta-peptide neurotoxicity in primary cortical culture. Exp Neurol 253:63–71. https://doi.org/10.1016/j.expneurol.2013.12.009
Chiang MC, Nicol CJB (2022) GSH-AuNP anti-oxidative stress, ER stress and mitochondrial dysfunction in amyloid-beta peptide-treated human neural stem cells. Free Radic Biol Med 187:185–201. https://doi.org/10.1016/j.freeradbiomed.2022.05.025
Davis CK, Vemuganti R (2022) Antioxidant therapies in traumatic brain injury. Neurochem Int 152:105255. https://doi.org/10.1016/j.neuint.2021.105255
Deng C, Yi R, Fei M, Li T, Han Y, Wang H (2021) Naringenin attenuates endoplasmic reticulum stress, reduces apoptosis, and improves functional recovery in experimental traumatic brain injury. Brain Res 1769:147591. https://doi.org/10.1016/j.brainres.2021.147591
Du Y, Ma X, Ma L, Li S, Zheng J, Lv J, Cui L, Lv J (2020) Inhibition of microRNA-148b-3p alleviates oxygen-glucose deprivation/reoxygenation-induced apoptosis and oxidative stress in HT22 hippocampal neuron via reinforcing Sestrin2/Nrf2 signalling. Clin Exp Pharmacol Physiol 47(4):561–570. https://doi.org/10.1111/1440-1681.13231
Fatima MT, Hasan M, Abdelsalam SS, Sivaraman SK, El-Gamal H, Zahid MA, Elrayess MA, Korashy HM, Zeidan A, Parray AS, Agouni A (2021) Sestrin2 suppression aggravates oxidative stress and apoptosis in endothelial cells subjected to pharmacologically induced endoplasmic reticulum stress. Eur J Pharmacol 907:174247. https://doi.org/10.1016/j.ejphar.2021.174247
Gao A, Li F, Zhou Q, Chen L (2020) Sestrin2 as a potential therapeutic target for Cardiovascular Diseases. Pharmacol Res 159:104990. https://doi.org/10.1016/j.phrs.2020.104990
Gao C, Chen X, Xu H, Guo H, Zheng L, Yan Y, Ren Z, Luo C, Gao Y, Wang Z, Tao L, Wang T (2022) Restraint stress delays the recovery of neurological impairments and exacerbates brain damages through activating endoplasmic reticulum stress-mediated Neurodegeneration/Autophagy/Apopotosis post Moderate Traumatic Brain Injury. Mol Neurobiol 59(3):1560–1576. https://doi.org/10.1007/s12035-022-02735-4
Han D, Kim H, Kim S, Le QA, Han SY, Bae J, Shin HW, Kang HG, Han KH, Shin J, Park HW (2022) Sestrin2 protects against cholestatic liver injury by inhibiting endoplasmic reticulum stress and NLRP3 inflammasome-mediated pyroptosis. Exp Mol Med 54(3):239–251. https://doi.org/10.1038/s12276-022-00737-9
Hay N (2008) p53 strikes mTORC1 by employing sestrins. Cell Metab 8(3):184–185. https://doi.org/10.1016/j.cmet.2008.08.010
He T, Li W, Song Y, Li Z, Tang Y, Zhang Z, Yang GY (2020) Sestrin2 regulates microglia polarization through mTOR-mediated autophagic flux to attenuate inflammation during experimental brain ischemia. J Neuroinflammation 17(1):329. https://doi.org/10.1186/s12974-020-01987-y
Hetzer SM, Guilhaume-Correa F, Day D, Bedolla A, Evanson NK (2021) Traumatic Optic Neuropathy is Associated with Visual Impairment, Neurodegeneration, and endoplasmic reticulum stress in adolescent mice. Cells 10(5):996. https://doi.org/10.3390/cells10050996
Holczer M, Hajdú B, Lőrincz T, Szarka A, Bánhegyi G, Kapuy O (2019) A double negative Feedback Loop between mTORC1 and AMPK Kinases Guarantees Precise Autophagy induction upon Cellular stress. Int J Mol Sci 20(22):5543. https://doi.org/10.3390/ijms20225543
Hou YS, Guan JJ, Xu HD, Wu F, Sheng R, Qin ZH (2015) Sestrin2 protects dopaminergic cells against Rotenone Toxicity through AMPK-Dependent Autophagy activation. Mol Cell Biol 35(16):2740–2751. https://doi.org/10.1128/MCB.00285-15
Hsieh YH, Chao AC, Lin YC, Chen SD, Yang DI (2021) The p53/NF-kappaB-dependent induction of sestrin2 by amyloid-beta peptides exerts antioxidative actions in neurons. Free Radic Biol Med 169:36–61. https://doi.org/10.1016/j.freeradbiomed.2021.04.004
Jang SK, Hong SE, Lee DH, Kim JY, Kim JY, Ye SK, Hong J, Park IC, Jin HO (2021) Inhibition of mTORC1 through ATF4-induced REDD1 and Sestrin2 expression by Metformin. BMC Cancer 21(1):803. https://doi.org/10.1186/s12885-021-08346-x
Jia Y, Zheng Z, Yang Y, Zou M, Li J, Wang L, Guan M, Xue Y (2019) MiR-4756 promotes albumin-induced renal tubular epithelial cell epithelial-to-mesenchymal transition and endoplasmic reticulum stress via targeting Sestrin2. J Cell Physiol 234(3):2905–2915. https://doi.org/10.1002/jcp.27107
Jiang C, Bi C, Jiang X, Tian T, Huang X, Wang C, Fernandez MR, Iqbal J, Chan WC, McKeithan TW, Lewis RE, Fu K (2019) The miR-17 ~ 92 cluster activates mTORC1 in mantle cell Lymphoma by targeting multiple regulators in the STK11/AMPK/TSC/mTOR pathway. Br J Haematol 185(3):616–620. https://doi.org/10.1111/bjh.15591
Kou JJ, Shi JZ, He YY, Hao JJ, Zhang HY, Luo DM, Song JK, Yan Y, Xie XM, Du GH, Pang XB (2022) Luteolin alleviates cognitive impairment in Alzheimer’s Disease mouse model via inhibiting endoplasmic reticulum stress-dependent neuroinflammation. Acta Pharmacol Sin 43(4):840–849. https://doi.org/10.1038/s41401-021-00702-8
Krishan S, Sahni S, Richardson DR (2020) The anti-tumor agent, Dp44mT, promotes nuclear translocation of TFEB via inhibition of the AMPK-mTORC1 axis. Biochim Biophys Acta Mol Basis Dis 1866(12):165970. https://doi.org/10.1016/j.bbadis.2020.165970
Lee HY, Lee GH, Yoon Y, Chae HJ (2019) Rhus verniciflua and Eucommia ulmoides protects against high-Fat Diet-Induced hepatic steatosis by enhancing Anti-oxidation and AMPK activation. Am J Chin Med 47(6):1253–1270. https://doi.org/10.1142/S0192415X19500642
Lee S, Shin J, Hong Y, Shin SM, Shin HW, Shin J, Lee SK, Park HW (2020) Sestrin2 alleviates palmitate-induced endoplasmic reticulum stress, apoptosis, and defective invasion of human trophoblast cells. Am J Reprod Immunol 83(4):e13222. https://doi.org/10.1111/aji.13222
Li H, Min Q, Ouyang C, Lee J, He C, Zou MH, Xie Z (2014) AMPK activation prevents excess nutrient-induced hepatic lipid accumulation by inhibiting mTORC1 signaling and endoplasmic reticulum stress response. Biochim Biophys Acta 1842(9):1844–1854. https://doi.org/10.1016/j.bbadis.2014.07.002
Li Y, Wu J, Yu S, Zhu J, Zhou Y, Wang P, Li L, Zhao Y (2020) Sestrin2 promotes angiogenesis to alleviate brain injury by activating Nrf2 through regulating the interaction between p62 and Keap1 following photothrombotic Stroke in rats. Brain Res 1745:146948. https://doi.org/10.1016/j.brainres.2020.146948
Li HQ, Xia SN, Xu SY, Liu PY, Gu Y, Bao XY, Xu Y, Cao X (2021a) γ-Glutamylcysteine alleviates ischemic Stroke-Induced neuronal apoptosis by inhibiting ROS-Mediated endoplasmic reticulum stress. Oxid Med Cell Longev 2021:2961079. https://doi.org/10.1155/2021/2961079
Li Y, Zhang J, Zhou K, Xie L, Xiang G, Fang M, Han W, Wang X, Xiao J (2021b) Elevating sestrin2 attenuates endoplasmic reticulum stress and improves functional recovery through autophagy activation after spinal cord injury. Cell Biol Toxicol 37(3):401–419. https://doi.org/10.1007/s10565-020-09550-4
Li G, Liang R, Lian Y, Zhou Y (2022a) Circ_0002945 functions as a competing endogenous RNA to promote Aβ25-35-induced endoplasmic reticulum stress and apoptosis in SK-N-SH cells and human primary neurons. Brain Res 1785:147878. https://doi.org/10.1016/j.brainres.2022.147878
Li L, Luo Q, Shang B, Yang X, Zhang Y, Pan Q, Wu N, Tang W, Du D, Sun X, Jiang L (2022b) Selective activation of cannabinoid receptor-2 reduces white matter injury via PERK signaling in a rat model of traumatic brain injury. Exp Neurol 347:113899. https://doi.org/10.1016/j.expneurol.2021.113899
Li X, Cheng Y, Qin Y, Gao H, Wang G, Song H, Wang Y, Cai B (2022c) Chrysophanol exerts neuroprotective effects via interfering with endoplasmic reticulum stress apoptotic pathways in cell and animal models of Alzheimer’s Disease. J Pharm Pharmacol 74(1):32–40. https://doi.org/10.1093/jpp/rgab148
Liu S, Jin R, Xiao AY, Chen R, Li J, Zhong W, Feng X, Li G (2019) Induction of neuronal PI3Kγ contributes to endoplasmic reticulum stress and long-term functional impairment in a murine model of traumatic brain Injury. Neurotherapeutics 16(4):1320–1334. https://doi.org/10.1007/s13311-019-00748-x
Liu J, Li Y, Mei C, Ning X, Pang J, Gu L, Wu L (2020) Phytic acid exerts protective effects in cerebral ischemia-reperfusion injury by activating the anti-oxidative protein sestrin2. Biosci Biotechnol Biochem 84(7):1401–1408. https://doi.org/10.1080/09168451.2020.1754158
Liu T, Li T, Chen X, Li Z, Feng M, Yao W, Wan L, Zhang C, Zhang Y (2021a) EETs/sEHi alleviates nociception by blocking the crosslink between endoplasmic reticulum stress and neuroinflammation in a central poststroke pain model. J Neuroinflammation 18(1):211. https://doi.org/10.1186/s12974-021-02255-3
Liu X, Li M, Zhu J, Huang W, Song J (2021b) Sestrin2 protects against traumatic brain injury by reinforcing the activation of Nrf2 signaling. Hum Exp Toxicol 40(7):1095–1111. https://doi.org/10.1177/0960327120984224
Loayza-Puch F, Drost J, Rooijers K, Lopes R, Elkon R, Agami R (2013) p53 induces transcriptional and translational programs to suppress cell proliferation and growth. Genome Biol 14(4):R32. https://doi.org/10.1186/gb-2013-14-4-r32
Lucke-Wold BP, Logsdon AF, Turner RC, Huber JD, Rosen CL (2017) Endoplasmic reticulum stress modulation as a target for ameliorating effects of Blast Induced Traumatic Brain Injury. J Neurotrauma 34(S1):S62–S70. https://doi.org/10.1089/neu.2016.4680
Luo J, Odaka Y, Huang Z, Cheng B, Liu W, Li L, Shang C, Zhang C, Wu Y, Luo Y, Yang S, Houghton PJ, Guo X, Huang S (2021) Dihydroartemisinin inhibits mTORC1 signaling by activating the AMPK pathway in Rhabdomyosarcoma Tumor cells. Cells 10(6):1363. https://doi.org/10.3390/cells10061363
Manifava M, Smith M, Rotondo S, Walker S, Niewczas I, Zoncu R, Clark J, Ktistakis NT (2016) Dynamics of mTORC1 activation in response to amino acids. Elife 5:e19960. https://doi.org/10.7554/eLife.19960
Medinas DB, Rozas P, Martínez Traub F, Woehlbier U, Brown RH, Bosco DA, Hetz C (2018) Endoplasmic reticulum stress leads to accumulation of wild-type SOD1 aggregates associated with sporadic Amyotrophic Lateral Sclerosis. Proc Natl Acad Sci U S A 115(32):8209–8214. https://doi.org/10.1073/pnas.1801109115
Najafov A, Luu HS, Mookhtiar AK, Mifflin L, Xia HG, Amin PP, Ordureau A, Wang H, Yuan J (2021) RIPK1 promotes Energy sensing by the mTORC1 pathway. Mol Cell 81(2):370–385e7. https://doi.org/10.1016/j.molcel.2020.11.008
Oh HJ, Lee S, Park PH (2020) ER stress contributes to autophagy induction by adiponectin in macrophages: implication in cell survival and suppression of inflammatory response. Cytokine 127:154959. https://doi.org/10.1016/j.cyto.2019.154959
Park HJ, Yang SG, Koo DB (2022) SESN2/NRF2 signaling activates as a direct downstream regulator of the PERK pathway against endoplasmic reticulum stress to improve the in vitro maturation of porcine oocytes. Free Radic Biol Med 178:413–427. https://doi.org/10.1016/j.freeradbiomed.2021.12.258
Pasha M, Eid AH, Eid AA, Gorin Y, Munusamy S (2017) Sestrin2 as a Novel Biomarker and Therapeutic Target for various Diseases. Oxid Med Cell Longev 2017:3296294. https://doi.org/10.1155/2017/3296294
Pham DV, Raut PK, Pandit M, Chang JH, Katila N, Choi DY, Jeong JH, Park PH (2020) Globular adiponectin inhibits Breast Cancer Cell Growth through Modulation of Inflammasome activation: critical role of Sestrin2 and AMPK Signaling. Cancers (Basel) 12(3):613. https://doi.org/10.3390/cancers12030613
Rai N, Upadhyay AD, Goyal V, Dwivedi S, Dey AB, Dey S (2020) Sestrin2 as serum protein marker and potential therapeutic target for Parkinson’s Disease. J Gerontol A Biol Sci Med Sci 75(4):690–695. https://doi.org/10.1093/gerona/glz234
Reddy K, Cusack CL, Nnah IC, Khayati K, Saqcena C, Huynh TB, Noggle SA, Ballabio A, Dobrowolski R (2016) Dysregulation of nutrient sensing and CLEARance in Presenilin Deficiency. Cell Rep 14(9):2166–2179. https://doi.org/10.1016/j.celrep.2016.02.006
Rinaldi L, Sepe M, Delle Donne R, Conte K, Arcella A, Borzacchiello D, Amente S, De Vita F, Porpora M, Garbi C, Oliva MA, Procaccini C, Faicchia D, Matarese G, Zito Marino F, Rocco G, Pignatiello S, Franco R, Insabato L, Majello B, Feliciello A (2017) Mitochondrial AKAP1 supports mTOR pathway and Tumor growth. Cell Death Dis 8(6):e2842. https://doi.org/10.1038/cddis.2017.241
Ro SH, Xue X, Ramakrishnan SK, Cho CS, Namkoong S, Jang I, Semple IA, Ho A, Park HW, Shah YM, Lee JH (2016) Tumor suppressive role of sestrin2 during Colitis and colon carcinogenesis. Elife 5:e12204. https://doi.org/10.7554/eLife.12204
Sawa R, Wake I, Yamamoto Y, Okimura Y (2021) The involvement of Sestrin2 in the effect of IGF-I and leucine on mTROC1 activity in C2C12 and L6 myocytes. Growth Horm IGF Res 59:101406. https://doi.org/10.1016/j.ghir.2021.101406
Sawa R, Ohnishi A, Ohno M, Nagata M, Wake I, Okimura Y (2022) Specific amino acids regulate Sestrin2 mRNA and protein levels in an ATF4-dependent manner in C2C12 myocytes. Biochim Biophys Acta Gen Subj 1866(9):130174. https://doi.org/10.1016/j.bbagen.2022.130174
Saxton RA, Knockenhauer KE, Wolfson RL, Chantranupong L, Pacold ME, Wang T, Schwartz TU, Sabatini DM (2016) Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway. Science. 351(6268):53–58. https://doi.org/10.1126/science.aad2087
Sen T, Saha P, Gupta R, Foley LM, Jiang T, Abakumova OS, Hitchens TK, Sen N (2020) Aberrant ER stress Induced Neuronal-IFNβ elicits White Matter Injury due to Microglial activation and T-Cell infiltration after TBI. J Neurosci 40(2):424–446. https://doi.org/10.1523/JNEUROSCI.0718-19.2019
Sengupta S, Giaime E, Narayan S, Hahm S, Howell J, O’Neill D, Vlasuk GP, Saiah E (2019) Discovery of NV-5138, the first selective brain mTORC1 activator. Sci Rep 9(1):4107. https://doi.org/10.1038/s41598-019-40693-5
Shi M, Chen F, Chen Z, Yang W, Yue S, Zhang J, Chen X (2021) Sigma-1 receptor: a potential therapeutic target for traumatic brain Injury. Front Cell Neurosci 15:685201. https://doi.org/10.3389/fncel.2021.685201
Shi M, Liu L, Min X, Mi L, Chai Y, Chen F, Wang J, Yue S, Zhang J, Deng Q, Chen X (2022) Activation of Sigma-1 receptor alleviates ER-Associated Cell Death and Microglia activation in traumatically injured mice. J Clin Med 11(9):2348. https://doi.org/10.3390/jcm11092348
Shin YH, Cho H, Choi BY, Kim J, Ha J, Suh SW, Park SB (2021) Phenotypic Discovery of Neuroprotective agents by Regulation of Tau Proteostasis via stress-responsive activation of PERK Signaling. Angew Chem Int Ed Engl 60(4):1831–1838. https://doi.org/10.1002/anie.202013915
Sinha P, Verma B, Ganesh S (2021) Trehalose ameliorates Seizure susceptibility in Lafora Disease Mouse models by suppressing neuroinflammation and endoplasmic reticulum stress. Mol Neurobiol 58(3):1088–1101. https://doi.org/10.1007/s12035-020-02170-3
Sun D, Wang J, Liu X, Fan Y, Yang M, Zhang J (2020) Dexmedetomidine attenuates endoplasmic reticulum stress-induced apoptosis and improves neuronal function after traumatic brain injury in mice. Brain Res 1732:146682. https://doi.org/10.1016/j.brainres.2020.146682
Sun G, Zhao Z, Lang J, Sun B, Zhao Q (2022) Nrf2 loss of function exacerbates endoplasmic reticulum stress-induced apoptosis in TBI mice. Neurosci Lett 770:136400. https://doi.org/10.1016/j.neulet.2021.136400
Suryawan A, Davis TA (2018) Amino acid- and insulin-Induced activation of mTORC1 in neonatal piglet skeletal muscle involves Sestin2-GATOR2, rag A/C-mTOR, and RHEB-mTOR complex formation. J Nutr 148(6):825–833. https://doi.org/10.1093/jn/nxy044
Tan HP, Guo Q, Hua G, Chen JX, Liang JC (2018) Inhibition of endoplasmic reticulum stress alleviates secondary injury after traumatic brain injury. Neural Regen Res 13(5):827–836. https://doi.org/10.4103/1673-5374.232477
Tang Z, Wei X, Li T, Wang W, Wu H, Dong H, Liu Y, Wei F, Shi L, Li X, Guo Z, Xiao X (2021) Sestrin2-Mediated Autophagy contributes to Drug Resistance via endoplasmic reticulum stress in human osteosarcoma. Front Cell Dev Biol 9:722960. https://doi.org/10.3389/fcell.2021.722960
Tian X, Gao Y, Zhong M, Kong M, Zhao L, Feng Z, Sun Q, He J, Liu X (2022) The association between serum Sestrin2 and the risk of coronary Heart Disease in patients with type 2 Diabetes Mellitus. BMC Cardiovasc Disord 22(1):281. https://doi.org/10.1186/s12872-022-02727-1
Wang P, Zhao Y, Li Y, Wu J, Yu S, Zhu J, Li L, Zhao Y (2019) Sestrin2 overexpression attenuates focal cerebral ischemic injury in rat by increasing Nrf2/HO-1 pathway-mediated angiogenesis. Neuroscience 410:140–149. https://doi.org/10.1016/j.neuroscience.2019.05.005
Wang C, Cai X, Wang R, Zhai S, Zhang Y, Hu W, Zhang Y, Wang D (2020a) Neuroprotective effects of verbascoside against Alzheimer’s Disease via the relief of endoplasmic reticulum stress in Aβ-exposed U251 cells and APP/PS1 mice. J Neuroinflammation 17(1):309. https://doi.org/10.1186/s12974-020-01976-1
Wang LX, Zhu XM, Luo YN, Wu Y, Dong N, Tong YL, Yao YM (2020b) Sestrin2 protects dendritic cells against endoplasmic reticulum stress-related apoptosis induced by high mobility group box-1 protein. Cell Death Dis 11(2):125. https://doi.org/10.1038/s41419-020-2324-4
Wang DY, Hong MY, Pei J, Gao YH, Zheng Y, Xu X (2021a) ER stress mediated-autophagy contributes to neurological dysfunction in traumatic brain injury via the ATF6 UPR signaling pathway. Mol Med Rep 23(4):247. https://doi.org/10.3892/mmr.2021.11886
Wang LX, Ren C, Yao RQ, Luo YN, Yin Y, Wu Y, Dong N, Zhu XM, Yao YM (2021b) Sestrin2 protects against lethal sepsis by suppressing the pyroptosis of dendritic cells. Cell Mol Life Sci 78(24):8209–8227. https://doi.org/10.1007/s00018-021-03970-z
Wang D, Xu C, Yang W, Chen J, Ou Y, Guan Y, Guan J, Liu Y (2022) E3 ligase RNF167 and deubiquitinase STAMBPL1 modulate mTOR and cancer progression. Mol Cell 82(4):770–784e9. https://doi.org/10.1016/j.molcel.2022.01.002
Wolfson RL, Sabatini DM (2017) The Dawn of the age of amino acid sensors for the mTORC1 pathway. Cell Metab 26(2):301–309. https://doi.org/10.1016/j.cmet.2017.07.001
Wu C, Du M, Yu R, Cheng Y, Wu B, Fu J, Tan W, Zhou Q, Balawi E, Liao ZB (2022) A novel mechanism linking ferroptosis and endoplasmic reticulum stress via the circPtpn14/miR-351-5p/5-LOX signaling in melatonin-mediated treatment of traumatic brain injury. Free Radic Biol Med 178:271–294. https://doi.org/10.1016/j.freeradbiomed.2021.12.007
Xu F, Du W, Zou Q, Wang Y, Zhang X, Xing X, Li Y, Zhang D, Wang H, Zhang W, Hu X, Liu X, Liu X, Zhang S, Yu J, Fang J, Li F, Zhou Y, Yue T, Mi N, Deng H, Zou P, Chen X, Yang X, Yu L (2021) COPII mitigates ER stress by promoting formation of ER whorls. Cell Res 31(2):141–156. https://doi.org/10.1038/s41422-020-00416-2
Yakhine-Diop SMS, Rodríguez-Arribas M, Canales-Cortés S, Martínez-Chacón G, Uribe-Carretero E, Blanco-Benítez M, Duque-González G, Paredes-Barquero M, Alegre-Cortés E, Climent V, Aiastui A, López de Munain A, Bravo-San Pedro JM, Niso-Santano M, Fuentes JM, González-Polo RA (2022) The parkinsonian LRRK2 R1441G mutation shows macroautophagy-mitophagy dysregulation concomitant with endoplasmic reticulum stress. Cell Biol Toxicol 38(5):889–911. https://doi.org/10.1007/s10565-021-09617-w
Yang X, Xue P, Yuan M, Xu X, Wang C, Li W, Machens HG, Chen Z (2021a) SESN2 protects against denervated muscle atrophy through unfolded protein response and mitophagy. Cell Death Dis 12(9):805. https://doi.org/10.1038/s41419-021-04094-9
Yang Y, Guo G, Zhou W, Ge Y, Fan Z, Liu Q, Gao Y (2021b) Sestrin2 protects against bavachin induced ER stress through AMPK/mTORC1 signaling pathway in HepG2 cells. J Pharmacol Sci 145(2):175–186. https://doi.org/10.1016/j.jphs.2020.11.012
Yang J, Guo Q, Wang L, Yu S (2023a) POU domain class 2 transcription factor 2 inhibits ferroptosis in Cerebral Ischemia Reperfusion Injury by activating Sestrin2. Neurochem Res 48(2):658–670. https://doi.org/10.1007/s11064-022-03791-x
Yang Y, Ding H, Yang C, Wu J, Bao Y, Lan S, Zhou L, Zhou L, Liu B, Hong T, Wan X, Wu X (2023b) Sestrin2 provides cerebral protection through activation of Nrf2 signaling in microglia following subarachnoid Hemorrhage. Front Immunol 14:1089576. https://doi.org/10.3389/fimmu.2023.1089576
Yao RQ, Ren C, Xia ZF, Yao YM (2021) Organelle-specific autophagy in inflammatory Diseases: a potential therapeutic target underlying the quality control of multiple organelles. Autophagy 17(2):385–401. https://doi.org/10.1080/15548627.2020.1725377
Ye J, Palm W, Peng M, King B, Lindsten T, Li MO, Koumenis C, Thompson CB (2015) GCN2 sustains mTORC1 suppression upon amino acid deprivation by inducing Sestrin2. Genes Dev 29(22):2331–2336. https://doi.org/10.1101/gad.269324.115
Yue J, Wei YJ, Yang XL, Liu SY, Yang H, Zhang CQ (2020) NLRP3 inflammasome and endoplasmic reticulum stress in the epileptogenic zone in temporal lobe Epilepsy: molecular insights into their interdependence. Neuropathol Appl Neurobiol 46(7):770–785. https://doi.org/10.1111/nan.12621
Zeng N, D’Souza RF, Mitchell CJ, Cameron-Smith D (2018) Sestrins are differentially expressed with age in the skeletal muscle of men: a cross-sectional analysis. Exp Gerontol 110:23–34. https://doi.org/10.1016/j.exger.2018.05.006
Zeng N, D’Souza RF, MacRae CL, Figueiredo VC, Pileggi CA, Markworth JF, Merry TL, Cameron-Smith D, Mitchell CJ (2021) Daily protein supplementation attenuates immobilization-induced blunting of postabsorptive muscle mTORC1 activation in middle-aged men. Am J Physiol Cell Physiol 320(4):C591–C601. https://doi.org/10.1152/ajpcell.00284.2020
Zhang LL, Zhang ZJ (2018) Sestrin2 aggravates oxidative stress of neurons by decreasing the expression of Nrf2. Eur Rev Med Pharmacol Sci 22(11):3493–3501. https://doi.org/10.26355/eurrev_201806_15176
Zhang N, Liao HH, Feng H, Mou SQ, Li WJ, Aiyasiding X, Lin Z, Ding W, Zhou ZY, Yan H, Chen S, Tang QZ (2021) Knockout of AMPKα2 blocked the Protection of Sestrin2 overexpression against Cardiac Hypertrophy Induced by pressure overload. Front Pharmacol 12:716884. https://doi.org/10.3389/fphar.2021.716884
Zhou D, Zhan C, Zhong Q, Li S (2013) Upregulation of sestrin-2 expression via P53 protects against 1-methyl-4-phenylpyridinium (MPP+) neurotoxicity. J Mol Neurosci 51(3):967–975. https://doi.org/10.1007/s12031-013-0081-x
Zong Y, Zhang CS, Li M, Wang W, Wang Z, Hawley SA, Ma T, Feng JW, Tian X, Qi Q, Wu YQ, Zhang C, Ye Z, Lin SY, Piao HL, Hardie DG, Lin SC (2019) Hierarchical activation of compartmentalized pools of AMPK depends on severity of nutrient or energy stress. Cell Res 29(6):460–473. https://doi.org/10.1038/s41422-019-0163-6
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
XL designed the study. YZ, YZ, BO.A.B, MH and XL prepared the first draft of the manuscript. YZ, BO.A.B, YZ, MH and XL revised the manuscript. All authors approved the final paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agreed to publish this article.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Y., Zhang, Y., Botchway, B.O. et al. Sestrin2 can alleviate endoplasmic reticulum stress to improve traumatic brain injury by activating AMPK/mTORC1 signaling pathway. Metab Brain Dis 39, 439–452 (2024). https://doi.org/10.1007/s11011-023-01323-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-023-01323-2