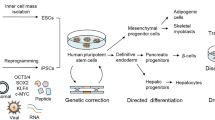
Abstract
Loss and functional failure of pancreatic β-cells results in disruption of glucose homeostasis and progression of diabetes. Although whole pancreas or pancreatic islet transplantation serves as a promising approach for β-cell replenishment and diabetes therapy, the severe scarcity of donor islets makes it unattainable for most diabetic patients. Stem cells, particularly induced pluripotent stem cells (iPSCs), are promising for the treatment of diabetes owing to their self-renewal capacity and ability to differentiate into functional β-cells. In this review, we first introduce the development of functional β-cells and their heterogeneity and then turn to highlight recent advances in the generation of β-cells from stem cells and their potential applications in disease modeling, drug discovery and clinical therapy. Finally, we have discussed the current challenges in developing stem cell-based therapeutic strategies for improving the treatment of diabetes. Although some significant technical hurdles remain, stem cells offer great hope for patients with diabetes and will certainly transform future clinical practice.




Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WHH, Zhang P, Bommer C, Kuo SH, Boyko EJJ, Magliano DJ (2022) IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Practice. https://doi.org/10.1016/j.diabres.2021.109119
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA, Assoc AD (2023) Classification and diagnosis of diabetes. Diabetes Care 46:S19–S40. https://doi.org/10.2337/dc23-S002
He SY, Yu XQ, Cui DX, Liu Y, Yang SS, Zhang HM, Hu WX, Su ZG (2023) Nuclear factor-Y mediates pancreatic beta-cell compensation by repressing reactive oxygen species-induced apoptosis under metabolic stress. Chin Med J 136:922–932. https://doi.org/10.1097/Cm9.0000000000002645
Dal Canto E, Ceriello A, Ryden L, Ferrini M, Hansen TB, Schnell O, Standl E, Beulens JWJ (2019) Diabetes as a cardiovascular risk factor: an overview of global trends of macro and micro vascular complications. Eur J Prev Cardiol 26:25–32. https://doi.org/10.1177/2047487319878371
Zhang XP, Deng D, Cui DX, Liu Y, He SY, Zhang HM, Xie YR, Yu XQ, Yang SS, Chen YL, Su ZG (2022) Cholesterol sulfate exerts protective effect on pancreatic beta-cells by regulating beta-cell mass and insulin secretion. Front Pharmacol. https://doi.org/10.3389/fphar.2022.840406
Zhang YJ, Guan QY, Liu Y, Zhang YW, Chen YL, Chen JL, Liu YL, Su ZG (2018) Regulation of hepatic gluconeogenesis by nuclear factor Y transcription factor in mice. J Biol Chem 293:7894–7904. https://doi.org/10.1074/jbc.RA117.000508
Ahmad E, Lim S, Lamptey R, Webb DR, Davies MJ (2022) Type 2 diabetes. Lancet 400:1803–1820. https://doi.org/10.1016/S0140-6736(22)01655-5
Satin LS, Soleimanpour SA, Walker EM (2021) New aspects of diabetes research and therapeutic development. Pharmacol Rev 73:1001–1015. https://doi.org/10.1124/pharmrev.120.000160
Liu Y, He SY, Zhou RX, Zhang XP, Yang SS, Deng D, Zhang CX, Yu XQ, Chen YL, Su ZG (2021) Nuclear factor-Y in mouse pancreatic beta-cells plays a crucial role in glucose homeostasis by regulating beta-cell mass and insulin secretion. Diabetes 70:1703–1716. https://doi.org/10.2337/db20-1238
Rorsman P, Ashcroft FM (2018) Pancreatic beta-cell electrical activity and insulin secretion: of mice and men. Physiol Rev 98:117–214. https://doi.org/10.1152/physrev.00008.2017
Herold KC, Vignali DAA, Cooke A, Bluestone JA (2013) Type 1 diabetes: translating mechanistic observations into effective clinical outcomes. Nat Rev Immunol 13:243–256. https://doi.org/10.1038/nri3422
Cui D, Feng X, Lei S, Zhang H, Hu W, Yang S, Yu X, Su Z (2024) Pancreatic beta-cell failure, clinical implications, and therapeutic strategies in type 2 diabetes. Chin Med J (Engl). https://doi.org/10.1097/CM9.0000000000003034
Zhang X, Yang S, Chen J, Su Z (2018) Unraveling the regulation of hepatic gluconeogenesis. Front Endocrinol (Lausanne) 9:802. https://doi.org/10.3389/fendo.2018.00802
Murphy R, Ellard S, Hattersley AT (2008) Clinical implications of a molecular genetic classification of monogenic beta-cell diabetes. Nat Clin Pract Endocrinol Metab 4:200–213. https://doi.org/10.1038/ncpendmet0778
Basile G, Qadir MMF, Mauvais-Jarvis F, Vetere A, Shoba V, Modell AE, Pastori RL, Russ HA, Wagner BK, Dominguez-Bendala J (2022) Emerging diabetes therapies: bringing back the?-cells. Molecular Metabolism. https://doi.org/10.1016/j.molmet.2022.101477
Gruessner AC, Gruessner RW (2016) Long-term outcome after pancreas transplantation: a registry analysis. Curr Opin Organ Transplant 21:377–385. https://doi.org/10.1097/MOT.0000000000000331
Stratta RJ, Farney AC, Fridell JA (2022) Analyzing outcomes following pancreas transplantation: definition of a failure or failure of a definition. Am J Transplant 22:1523–1526. https://doi.org/10.1111/ajt.17003
Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV (2000) Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 343:230–238. https://doi.org/10.1056/NEJM200007273430401
Marfil-Garza BA, Shapiro AMJ, Kin T (2021) Clinical islet transplantation: current progress and new frontiers. J Hepatobiliary Pancreat Sci 28:243–254. https://doi.org/10.1002/jhbp.891
Salib A, Cayabyab F, Yoshihara E (2022) Stem cell-derived islets for type 2 diabetes. Int J Mol Sci. https://doi.org/10.3390/ijms23095099
Liu G, David BT, Trawczynski M, Fessler RG (2020) Advances in pluripotent stem cells: history, mechanisms, technologies, and applications. Stem Cell Rev Rep 16:3–32. https://doi.org/10.1007/s12015-019-09935-x
Campbell JE, Newgard CB (2021) Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat Rev Mol Cell Biol 22:142–158. https://doi.org/10.1038/s41580-020-00317-7
Tritschler S, Theis FJ, Lickert H, Böttcher A (2017) Systematic single-cell analysis provides new insights into heterogeneity and plasticity of the pancreas. Mol Metab 6:974–990. https://doi.org/10.1016/j.molmet.2017.06.021
Roscioni SS, Migliorini A, Gegg M, Lickert H (2016) Impact of islet architecture on β-cell heterogeneity, plasticity and function. Nat Rev Endocrinol 12:695–709. https://doi.org/10.1038/nrendo.2016.147
Pan FC, Wright C (2011) Pancreas organogenesis: from bud to plexus to gland. Dev Dyn 240:530–565. https://doi.org/10.1002/dvdy.22584
Shih HP, Wang A, Sander M (2013) Pancreas organogenesis: from lineage determination to morphogenesis. Annu Rev Cell Dev Biol 29:81–105. https://doi.org/10.1146/annurev-cellbio-101512-122405
Collombat P, Mansouri A, Hecksher-Sorensen J, Serup P, Krull J, Gradwohl G, Gruss P (2003) Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev 17:2591–2603. https://doi.org/10.1101/gad.269003
Barsby T, Otonkoski T (2022) Maturation of beta cells: lessons from in vivo and in vitro models. Diabetologia 65:917–930. https://doi.org/10.1007/s00125-022-05672-y
Nishimura W, Iwasa H, Tumurkhuu M (2022) Role of the transcription Factor MAFA in the maintenance of pancreatic β-Cells. Int J Mole Sci. https://doi.org/10.3390/ijms23094478
Jennings RE, Berry AA, Strutt JP, Gerrard DT, Hanley NA (2015) Human pancreas development. Development 142:3126–3137. https://doi.org/10.1242/dev.120063
Nair G, Hebrok M (2015) Islet formation in mice and men: lessons for the generation of functional insulin-producing beta-cells from human pluripotent stem cells. Curr Opin Genet Dev 32:171–180. https://doi.org/10.1016/j.gde.2015.03.004
Russell R, Carnese PP, Hennings TG, Walker EM, Russ HA, Liu JS, Giacometti S, Stein R, Hebrok M (2020) Loss of the transcription factor MAFB limits β-cell derivation from human PSCs. Nat Commun 11:2742. https://doi.org/10.1038/s41467-020-16550-9
Arda HE, Li L, Tsai J, Torre EA, Rosli Y, Peiris H, Spitale RC, Dai C, Gu X, Qu K, Wang P, Wang J, Grompe M, Scharfmann R, Snyder MS, Bottino R, Powers AC, Chang HY, Kim SK (2016) Age-dependent pancreatic gene regulation reveals mechanisms governing human β cell function. Cell Metab 23:909–920. https://doi.org/10.1016/j.cmet.2016.04.002
Cyphert HA, Walker EM, Hang Y, Dhawan S, Haliyur R, Bonatakis L, Avrahami D, Brissova M, Kaestner KH, Bhushan A, Powers AC, Stein R (2019) Examining how the MAFB transcription factor affects islet β-cell function postnatally. Diabetes 68:337–348. https://doi.org/10.2337/db18-0903
Ma Z, Zhang X, Zhong W, Yi H, Chen X, Zhao Y, Ma Y, Song E, Xu T (2023) Deciphering early human pancreas development at the single-cell level. Nat Commun 14:5354. https://doi.org/10.1038/s41467-023-40893-8
Miranda MA, Macias-Velasco JF, Lawson HA (2021) Pancreatic (β-cell heterogeneity in health and diabetes: classes, sources, and subtypes. Am J Physiol-Endocrinol Metab 320:E716–E731. https://doi.org/10.1152/ajpendo.00649.2020
Giordano E, Bosco D, Cirulli V, Meda P (1991) Repeated glucose stimulation reveals distinct and lasting secretion patterns of individual rat pancreatic B cells. J Clin Invest 87:2178–2185. https://doi.org/10.1172/jci115251
Salinno C, Cota P, Bastidas-Ponce A, Tarquis-Medina M, Lickert H, Bakhti M (2019) β-cell maturation and identity in health and disease. Int J Mole Sci. https://doi.org/10.3390/ijms20215417
Dominguez-Gutierrez G, Xin YR, Gromada J (2019) Heterogeneity of human pancreatic beta-cells. Mol Metab 27:S7–S14. https://doi.org/10.1016/j.molmet.2019.06.015
Dorrell C, Schug J, Canaday PS, Russ HA, Tarlow BD, Grompe MT, Horton T, Hebrok M, Streeter PR, Kaestner KH, Grompe M (2016) Human islets contain four distinct subtypes of β cells. Nat Commun 7:11756. https://doi.org/10.1038/ncomms11756
Johnston NR, Mitchell RK, Haythorne E, Pessoa MP, Semplici F, Ferrer J, Piemonti L, Marchetti P, Bugliani M, Bosco D, Berishvili E, Duncanson P, Watkinson M, Broichhagen J, Trauner D, Rutter GA, Hodson DJ (2016) Beta cell hubs dictate pancreatic islet responses to glucose. Cell Metab 24:389–401. https://doi.org/10.1016/j.cmet.2016.06.020
Bader E, Migliorini A, Gegg M, Moruzzi N, Gerdes J, Roscioni SS, Bakhti M, Brandl E, Irmler M, Beckers J, Aichler M, Feuchtinger A, Leitzinger C, Zischka H, Wang-Sattler R, Jastroch M, Tschöp M, Machicao F, Staiger H, Häring HU, Chmelova H, Chouinard JA, Oskolkov N, Korsgren O, Speier S, Lickert H (2016) Identification of proliferative and mature β-cells in the islets of Langerhans. Nature 535:430. https://doi.org/10.1038/nature18624
Li X, Yang KY, Chan VW, Leung KT, Zhang XB, Wong AS, Chong CCN, Wang CC, Ku M, Lui KO (2020) Single-Cell RNA-Seq Reveals that CD9 Is a negative marker of glucose-responsive pancreatic beta-like cells derived from human pluripotent stem cells. Stem Cell Reports 15:1111–1126. https://doi.org/10.1016/j.stemcr.2020.09.009
Dror E, Fagnocchi L, Weqert V, Apostle S, Grimaldi B, Gruber T, Panzeri I, Heyne S, Hoffler KD, Kreiner V, Ching RG, Lu TTH, Semwal A, Johnson B, Senapati P, Lempradl A, Schones D, Imhof A, Shen H, Pospisilik JA (2023) Epigenetic dosage identifies two major and functionally distinct j3 cell subtypes. Cell Metab 35:821. https://doi.org/10.1016/j.cmet.2023.03.008
Aldous N, Moin AM, Abdelalim EM (2023) Pancreatic β-cell heterogeneity in adult human islets and stem cell-derived islets. Cell Mol Life Sci. https://doi.org/10.1007/s00018-023-04815-7
Sarkar A, Saha S, Paul A, Maji A, Roy P, Maity TK (2021) Understanding stem cells and its pivotal role in regenerative medicine. Life Sci. https://doi.org/10.1016/j.lfs.2021.119270
Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, Armstrong L, Djonov V, Lako M, Stojkovic M (2018) Ethical and safety issues of stem cell-based therapy. Int J Med Sci 15:36–45. https://doi.org/10.7150/ijms.21666
Keller GM (1995) In-vitro differentiation of embryonic stem-cells. Curr Opin Cell Biol 7:862–869. https://doi.org/10.1016/0955-0674(95)80071-9
Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R (2001) Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science 292:1389–1394. https://doi.org/10.1126/science.1058866
Rajagopal J, Anderson WJ, Kume S, Martinez OI, Melton DA (2003) Insulin staining of ES cell progeny from insulin uptake. Science 299:363–363
D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE (2005) Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol 23:1534–1541. https://doi.org/10.1038/nbt1163
D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE (2006) Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 24:1392–1401. https://doi.org/10.1038/nbt1259
Jiang JJ, Au M, Lu KH, Eshpeter A, Korbutt G, Fisk G, Majumdar AS (2007) Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells 25:1940–1953. https://doi.org/10.1634/stemcells.2006-0761
Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, O’Dwyer S, Quiskamp N, Mojibian M, Albrecht T, Yang YH, Johnson JD, Kieffer TJ (2014) Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 32:1121–1133. https://doi.org/10.1038/nbt.3033
Pagliuca FW, Millman JR, Gurtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA (2014) Generation of functional human pancreatic beta cells in vitro. Cell 159:428–439. https://doi.org/10.1016/j.cell.2014.09.040
Nair GG, Liu JS, Russ HA, Tran S, Saxton MS, Chen R, Juang C, Li ML, Nguyen VQ, Giacometti S, Puri S, Xing Y, Wang Y, Szot GL, Oberholzer J, Bhushan A, Hebrok M (2019) Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived beta cells. Nat Cell Biol 21:263–274. https://doi.org/10.1038/s41556-018-0271-4
Golchin A, Chatziparasidou A, Ranjbarvan P, Niknam Z, Ardeshirylajimi A (2021) Embryonic stem cells in clinical trials: current overview of developments and challenges. Adv Exp Med Biol 1312:19–37. https://doi.org/10.1007/5584_2020_592
Maxwell KG, Millman JR (2021) Applications of iPSC-derived beta cells from patients with diabetes. Cell Rep Med 2:100238. https://doi.org/10.1016/j.xcrm.2021.100238
Agrawal A, Narayan G, Gogoi R, Thummer RP (2021) Recent advances in the generation of β-cells from induced pluripotent stem cells as a potential cure for diabetes mellitus. Adv Exp Med Biol 1347:1–27. https://doi.org/10.1007/5584_2021_653
Loretelli C, Assi E, Seelam AJ, Ben Nasr M, Fiorina P (2020) Cell therapy for type 1 diabetes. Expert Opin Biol Ther 20:887–897. https://doi.org/10.1080/14712598.2020.1748596
Veres A, Faust AL, Bushnell HL, Engquist EN, Kenty JHR, Harb G, Poh YC, Sintov E, Gurtler M, Pagliuca FW, Peterson QP, Melton DA (2019) Charting cellular identity during human in vitro beta-cell differentiation. Nature 569:368. https://doi.org/10.1038/s41586-019-1168-5
Augsornworawat P, Maxwell KG, Velazco-Cruz L, Millman JR (2020) Single-Cell Transcriptome Profiling Reveals β Cell Maturation in Stem Cell-Derived Islets after Transplantation. Cell Rep 32:8. https://doi.org/10.1016/j.celrep.2020.108067
Velazco-Cruz L, Song J, Maxwell KG, Goedegebuure MM, Augsornworawat P, Hogrebe NJ, Millman JR (2019) Acquisition of dynamic function in human stem cell-derived beta cells. Stem Cell Reports 12:351–365. https://doi.org/10.1016/j.stemcr.2018.12.012
Hogrebe NJ, Augsornworawat P, Maxwell KG, Velazco-Cruz L, Millman JR (2020) Targeting the cytoskeleton to direct pancreatic differentiation of human pluripotent stem cells. Nat Biotechnol 38:460–470. https://doi.org/10.1038/s41587-020-0430-6
Maxwell KG, Augsornworawat P, Velazco-Cruz L, Kim MH, Asada R, Hogrebe NJ, Morikawa S, Urano F, Millman JR (2020) Gene-edited human stem cell-derived beta cells from a patient with monogenic diabetes reverse preexisting diabetes in mice. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aax9106
Yoshihara E, O’Connor C, Gasser E, Wei Z, Oh TG, Tseng TW, Wang D, Cayabyab F, Dai Y, Yu RT, Liddle C, Atkins AR, Downes M, Evans RM (2020) Immune-evasive human islet-like organoids ameliorate diabetes. Nature 586:606–611. https://doi.org/10.1038/s41586-020-2631-z
Parent AV, Ashe S, Nair GG, Li ML, Chavez J, Liu JS, Zhong YP, Streeter PR, Hebrok M (2022) Development of a scalable method to isolate subsets of stem cell-derived pancreatic islet cells. Stem Cell Reports 17:979–992. https://doi.org/10.1016/j.stemcr.2022.02.001
Sui L, Xin Y, Du Q, Georgieva D, Diedenhofen G, Haataja L, Su Q, Zuccaro MV, Kim J, Fu J, Xing Y, He Y, Baum D, Goland RS, Wang Y, Oberholzer J, Barbetti F, Arvan P, Kleiner S, Egli D (2021) Reduced replication fork speed promotes pancreatic endocrine differentiation and controls graft size. JCI Insight. https://doi.org/10.1172/jci.insight.141553
Shilleh AH, Beard S, Russ HA (2023) Enrichment of stem cell-derived pancreatic beta-like cells and controlled graft size through pharmacological removal of proliferating cells. Stem Cell Reports 18:1284–1294. https://doi.org/10.1016/j.stemcr.2023.05.010
Guan JY, Wang G, Wang JL, Zhang ZY, Fu Y, Cheng L, Meng GF, Lyu YL, Zhu JL, Li YQ, Wang YL, Liuyang SJ, Liu B, Yang ZR, He HJ, Zhong XX, Chen QJ, Zhang X, Sun SC, Lai WF, Shi Y, Liu LL, Wang LP, Li C, Lu SC, Deng HK (2022) Chemical reprogramming of human somatic cells to pluripotent stem cells. Nature 605:325. https://doi.org/10.1038/s41586-022-04593-5
Du YY, Liang Z, Wang S, Sun D, Wang XF, Liew SY, Lu SY, Wu SS, Jiang Y, Wang YQ, Zhang BY, Yu WH, Lu Z, Pu Y, Zhang Y, Long HT, Xiao SS, Liang R, Zhang ZY, Guan JY, Wang JL, Ren HX, Wei YL, Zhao JX, Sun SC, Liu TL, Meng GF, Wang L, Gu JB, Wang T, Liu YN, Li C, Tang C, Shen ZY, Peng XZ, Deng HK (2022) Human pluripotent stem-cell-derived islets ameliorate diabetes in non-human primates. Nat Med 28:2725. https://doi.org/10.1038/s41591-021-01645-7
Liang Z, Sun D, Lu SY, Lei ZJ, Wang SS, Luo ZF, Zhan JQ, Wu SS, Jiang Y, Lu Z, Sun SC, Shi YY, Long HT, Wei YL, Yu WH, Wang ZH, Yi LS, Zhang Y, Sun WY, Fang XF, Li YY, Lu SF, Lv JY, Sui WG, Shen ZY, Peng XZ, Du YY, Deng HK (2023) Implantation underneath the abdominal anterior rectus sheath enables effective and functional engraftment of stem-cell-derived islets. Nat Metab. https://doi.org/10.1038/s42255-022-00713-7
Ghoneim MA, Refaie AF, Elbassiouny BL, Gabr MM, Zakaria MM (2020) From mesenchymal stromal/stem cells to insulin-producing cells: progress and challenges. Stem Cell Rev Rep 16:1156–1172. https://doi.org/10.1007/s12015-020-10036-3
Wu X, Jiang J, Gu Z, Zhang J, Chen Y, Liu X (2020) Mesenchymal stromal cell therapies: immunomodulatory properties and clinical progress. Stem Cell Res Ther 11:345. https://doi.org/10.1186/s13287-020-01855-9
Berman DM, Willman MA, Han D, Kleiner G, Kenyon NM, Cabrera O, Karl JA, Wiseman RW, O’Connor DH, Bartholomew AM, Kenyon NS (2010) Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates. Diabetes 59:2558–2568. https://doi.org/10.2337/db10-0136
Tang YY, Zhou Y, Li HJ (2021) Advances in mesenchymal stem cell exosomes: a review. Stem Cell Res Ther. https://doi.org/10.1186/s13287-021-02138-7
Margiana R, Markov A, Zekiy AO, Hamza MU, Al-Dabbagh KA, Al-Zubaidi SH, Hameed NM, Ahmad I, Sivaraman R, Kzar HH, Al-Gazally ME, Mustafa YF, Siahmansouri H (2022) Clinical application of mesenchymal stem cell in regenerative medicine: a narrative review. Stem Cell Res Ther. https://doi.org/10.1186/s13287-022-03054-0
Kadam S, Muthyala S, Nair P, Bhonde R (2010) Human placenta-derived mesenchymal stem cells and islet-like cell clusters generated from these cells as a novel source for stem cell therapy in diabetes. Rev Diabet Stud 7:168–182. https://doi.org/10.1900/rds.2010.7.168
Zhang Y, Shen W, Hua J, Lei A, Lv C, Wang H, Yang C, Gao Z, Dou Z (2010) Pancreatic islet-like clusters from bone marrow mesenchymal stem cells of human first-trimester abortus can cure streptozocin-induced mouse diabetes. Rejuvenation Res 13:695–706. https://doi.org/10.1089/rej.2009.1016
Path G, Perakakis N, Mantzoros CS, Seufert J (2019) Stem cells in the treatment of diabetes mellitus - Focus on mesenchymal stem cells. Metabolism 90:1–15. https://doi.org/10.1016/j.metabol.2018.10.005
Eydian Z, Mohammad Ghasemi A, Ansari S, Kamali AN, Khosravi M, Momtaz S, Riki S, Rafighdoost L, Entezari Heravi R (2022) Differentiation of multipotent stem cells to insulin-producing cells for treatment of diabetes mellitus: bone marrow- and adipose tissue-derived cells comparison. Mol Biol Rep 49:3539–3548. https://doi.org/10.1007/s11033-022-07194-7
Cho J, D’Antuono M, Glicksman M, Wang J, Jonklaas J (2018) A review of clinical trials: mesenchymal stem cell transplant therapy in type 1 and type 2 diabetes mellitus. Am J Stem Cells 7:82–93
Millman JR, Xie C, Van Dervort A, Gürtler M, Pagliuca FW, Melton DA (2016) Generation of stem cell-derived β-cells from patients with type 1 diabetes. Nat Commun 7:11463. https://doi.org/10.1038/ncomms11463
Leite NC, Sintov E, Meissner TB, Brehm MA, Greiner DL, Harlan DM, Melton DA (2020) Modeling type 1 diabetes in vitro using human pluripotent stem cells. Cell Rep 32:107894. https://doi.org/10.1016/j.celrep.2020.107894
Shojima N, Yamauchi T (2023) Progress in genetics of type 2 diabetes and diabetic complications. J Diabetes Investig 14:503–515. https://doi.org/10.1111/jdi.13970
Zeng H, Guo M, Zhou T, Tan L, Chong CN, Zhang T, Dong X, Xiang JZ, Yu AS, Yue L, Qi Q, Evans T, Graumann J, Chen S (2016) An isogenic human ESC platform for functional evaluation of genome-wide-association-study-identified diabetes genes and drug discovery. Cell Stem Cell 19:326–340. https://doi.org/10.1016/j.stem.2016.07.002
Wen XJ, Yang YS (2017) Emerging roles of GLIS3 in neonatal diabetes, type 1 and type 2 diabetes. J Mol Endocrinol 58:R73–R85. https://doi.org/10.1530/Jme-16-0232
Amin S, Cook B, Zhou T, Ghazizadeh Z, Lis R, Zhang T, Khalaj M, Crespo M, Perera M, Xiang JZ, Zhu Z, Tomishima M, Liu C, Naji A, Evans T, Huangfu D, Chen S (2018) Discovery of a drug candidate for GLIS3-associated diabetes. Nat Commun 9:2681. https://doi.org/10.1038/s41467-018-04918-x
Hussain MA, Akalestou E, Song WJ (2016) Inter-organ communication and regulation of beta cell function. Diabetologia 59:659–667. https://doi.org/10.1007/s00125-015-3862-7
Picollet-D’hahan N, Zuchowska A, Lemeunier I, Le Gac S (2021) Multiorgan-on-a-Chip: a systemic approach to model and decipher inter-organ communication. Trends Biotechnol 39:788–810. https://doi.org/10.1016/j.tibtech.2020.11.014
Bauer S, Wennberg Huldt C, Kanebratt KP, Durieux I, Gunne D, Andersson S, Ewart L, Haynes WG, Maschmeyer I, Winter A, Ämmälä C, Marx U, Andersson TB (2017) Functional coupling of human pancreatic islets and liver spheroids on-a-chip: towards a novel human ex vivo type 2 diabetes model. Sci Rep 7:14620. https://doi.org/10.1038/s41598-017-14815-w
Yamashita-Sugahara Y, Matsumoto M, Ohtaka M, Nishimura K, Nakanishi M, Mitani K, Okazaki Y (2016) An inhibitor of fibroblast growth factor receptor-1 (FGFR1) promotes late-stage terminal differentiation from NGN3+ pancreatic endocrine progenitors. Sci Rep 6:35908. https://doi.org/10.1038/srep35908
Kimura A, Toyoda T, Nishi Y, Nasu M, Ohta A, Osafune K (2017) Small molecule AT7867 proliferates PDX1-expressing pancreatic progenitor cells derived from human pluripotent stem cells. Stem Cell Res 24:61–68. https://doi.org/10.1016/j.scr.2017.08.010
Korostylev A, Mahaddalkar PU, Keminer O, Hadian K, Schorpp K, Gribbon P, Lickert H (2017) A high-content small molecule screen identifies novel inducers of definitive endoderm. Mol Metab 6:640–650. https://doi.org/10.1016/j.molmet.2017.04.009
Zhou T, Kim TW, Chong CN, Tan L, Amin S, Badieyan ZS, Mukherjee S, Ghazizadeh Z, Zeng H, Guo M, Crespo M, Zhang T, Kenyon R, Robinson CL, Apostolou E, Wang H, Xiang JZ, Evans T, Studer L, Chen SB (2018) A hPSC-based platform to discover gene-environment interactions that impact human β-cell and dopamine neuron survival. Nat Commun. https://doi.org/10.1038/s41467-018-07201-1
Wei Z, Yoshihara E, He N, Hah N, Fan W, Pinto AFM, Huddy T, Wang Y, Ross B, Estepa G, Dai Y, Ding N, Sherman MH, Fang S, Zhao X, Liddle C, Atkins AR, Yu RT, Downes M, Evans RM (2018) Vitamin D switches BAF complexes to protect β cells. Cell 173:1135-1149.e15. https://doi.org/10.1016/j.cell.2018.04.013
Bashor CJ, Hilton IB, Bandukwala H, Smith DM, Veiseh O (2022) Engineering the next generation of cell-based therapeutics. Nat Rev Drug Discov 21:655–675. https://doi.org/10.1038/s41573-022-00476-6
Wang X, Gao M, Wang Y, Zhang Y (2022) The progress of pluripotent stem cell-derived pancreatic β-cells regeneration for diabetic therapy. Front Endocrinol (Lausanne) 13:927324. https://doi.org/10.3389/fendo.2022.927324
Ramzy A, Thompson DM, Ward-Hartstonge KA, Ivison S, Cook L, Garcia RV, Loyal J, Kim PTW, Warnock GL, Levings MK, Kieffer TJ (2021) Implanted pluripotent stem-cell-derived pancreatic endoderm cells secrete glucose-responsive C-peptide in patients with type 1 diabetes. Cell Stem Cell 28:2047-2061.e5. https://doi.org/10.1016/j.stem.2021.10.003
Shapiro AMJ, Thompson D, Donner TW, Bellin MD, Hsueh W, Pettus J, Wilensky J, Daniels M, Wang RM, Brandon EP, Jaiman MS, Kroon EJ, D’Amour KA, Foyt HL (2021) Insulin expression and C-peptide in type 1 diabetes subjects implanted with stem cell-derived pancreatic endoderm cells in an encapsulation device. Cell Rep Med. https://doi.org/10.1016/j.xcrm.2021.100466
Russ HA, Parent AV, Ringler JJ, Hennings TG, Nair GG, Shveygert M, Guo T, Puri S, Haataja L, Cirulli V, Blelloch R, Szot GL, Arvan P, Hebrok M (2015) Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J 34:1759–1772. https://doi.org/10.15252/embj.201591058
Agulnick AD, Ambruzs DM, Moorman MA, Bhoumik A, Cesario RM, Payne JK, Kelly JR, Haakmeester C, Srijemac R, Wilson AZ, Kerr J, Frazier MA, Kroon EJ, D’Amour KA (2015) Insulin-producing endocrine cells differentiated in vitro from human embryonic stem cells function in macroencapsulation devices in vivo. Stem Cells Transl Med 4:1214–1222. https://doi.org/10.5966/sctm.2015-0079
Nostro MC, Sarangi F, Yang C, Holland A, Elefanty AG, Stanley EG, Greiner DL, Keller G (2015) Efficient generation of NKX6-1+ pancreatic progenitors from multiple human pluripotent stem cell lines. Stem Cell Rep 4:591–604. https://doi.org/10.1016/j.stemcr.2015.02.017
Memon B, Karam M, Al-Khawaga S, Abdelalim EM (2018) Enhanced differentiation of human pluripotent stem cells into pancreatic progenitors co-expressing PDX1 and NKX6.1. Stem Cell Res Ther 9:15. https://doi.org/10.1186/s13287-017-0759-z
Desai T, Shea LD (2017) Advances in islet encapsulation technologies. Nat Rev Drug Discov 16:338–350. https://doi.org/10.1038/nrd.2016.232
Wu S, Wang L, Fang Y, Huang H, You X, Wu J (2021) Advances in encapsulation and delivery strategies for islet transplantation. Adv Healthc Mater 10:e2100965. https://doi.org/10.1002/adhm.202100965
Vaithilingam V, Bal S, Tuch BE (2017) Encapsulated islet transplantation: where do we stand? Rev Diabet Stud 14:51–78. https://doi.org/10.1900/rds.2017.14.51
Rafael E, Wernerson A, Arner P, Wu GS, Tibell A (1999) In vivo evaluation of glucose permeability of an immunoisolation device intended for islet transplantation: a novel application of the microdialysis technique. Cell Transplant 8:317–326. https://doi.org/10.1177/096368979900800302
Motté E, Szepessy E, Suenens K, Stangé G, Bomans M, Jacobs-Tulleneers-Thevissen D, Ling Z, Kroon E, Pipeleers D (2014) Composition and function of macroencapsulated human embryonic stem cell-derived implants: comparison with clinical human islet cell grafts. Am J Physiol Endocrinol Metab 307:E838–E846. https://doi.org/10.1152/ajpendo.00219.2014
Robert T, De Mesmaeker I, Stangé GM, Suenens KG, Ling Z, Kroon EJ, Pipeleers DG (2018) Functional beta cell mass from device-encapsulated HESC-derived pancreatic endoderm achieving metabolic control. Stem Cell Rep 10:739–750. https://doi.org/10.1016/j.stemcr.2018.01.040
Bruin JE, Rezania A, Xu J, Narayan K, Fox JK, O’Neil JJ, Kieffer TJ (2013) Maturation and function of human embryonic stem cell-derived pancreatic progenitors in macroencapsulation devices following transplant into mice. Diabetologia 56:1987–1998. https://doi.org/10.1007/s00125-013-2955-4
Zhang Q, Gonelle-Gispert C, Li Y, Geng Z, Gerber-Lemaire S, Wang Y, Buhler L (2022) Islet encapsulation: new developments for the treatment of type 1 diabetes. Front Immunol 13:869984. https://doi.org/10.3389/fimmu.2022.869984
Samojlik MM, Stabler CL (2021) Designing biomaterials for the modulation of allogeneic and autoimmune responses to cellular implants in type 1 diabetes. Acta Biomater 133:87–101. https://doi.org/10.1016/j.actbio.2021.05.039
Teramura Y, Iwata H (2009) Islet encapsulation with living cells for improvement of biocompatibility. Biomaterials 30:2270–2275. https://doi.org/10.1016/j.biomaterials.2009.01.036
Cui W, Khan KM, Ma X, Chen G, Desai CS (2020) Human amniotic epithelial cells and human amniotic membrane as a vehicle for islet cell transplantation. Trans Proc 52:982–986. https://doi.org/10.1016/j.transproceed.2020.01.022
Sabek OM, Ferrati S, Fraga DW, Sih J, Zabre EV, Fine DH, Ferrari M, Gaber AO, Grattoni A (2013) Characterization of a nanogland for the autotransplantation of human pancreatic islets. Lab Chip 13:3675–3688. https://doi.org/10.1039/c3lc50601k
Barra JM, Kozlovskaya V, Burnette KS, Banerjee RR, Fraker CA, Kharlampieva E, Tse HM (2023) Localized cytotoxic T cell-associated antigen 4 and antioxidant islet encapsulation alters macrophage signaling and induces regulatory and anergic T cells to enhance allograft survival. Am J Trans 23:498–511. https://doi.org/10.1016/j.ajt.2023.01.007
Elisseeff J, Badylak SF, Boeke JD (2021) Immune and genome engineering as the future of transplantable tissue. N Engl J Med 385:2451–2462. https://doi.org/10.1056/NEJMra1913421
Ghoneim MA, Gabr MM, El-Halawani SM, Refaie AF (2024) Current status of stem cell therapy for type 1 diabetes: a critique and a prospective consideration. Stem Cell Res Ther 15:23. https://doi.org/10.1186/s13287-024-03636-0
Deuse T, Hu X, Gravina A, Wang D, Tediashvili G, De C, Thayer WO, Wahl A, Garcia JV, Reichenspurner H, Davis MM, Lanier LL, Schrepfer S (2019) Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat Biotechnol 37:252–258. https://doi.org/10.1038/s41587-019-0016-3
Han X, Wang M, Duan S, Franco PJ, Kenty JH, Hedrick P, Xia Y, Allen A, Ferreira LMR, Strominger JL, Melton DA, Meissner TB, Cowan CA (2019) Generation of hypoimmunogenic human pluripotent stem cells. Proc Natl Acad Sci U S A 116:10441–10446. https://doi.org/10.1073/pnas.1902566116
Gerace D, Zhou Q, Kenty JH, Veres A, Sintov E, Wang X, Boulanger KR, Li H, Melton DA (2023) Engineering human stem cell-derived islets to evade immune rejection and promote localized immune tolerance. Cell Rep Med 4:100879. https://doi.org/10.1016/j.xcrm.2022.100879
Santini-Gonzalez J, Castro-Gutierrez R, Becker MW, Rancourt C, Russ HA, Phelps EA (2022) Human stem cell derived beta-like cells engineered to present PD-L1 improve transplant survival in NOD mice carrying human HLA class I. Front Endocrinol (Lausanne) 13:989815. https://doi.org/10.3389/fendo.2022.989815
Hu X, Gattis C, Olroyd AG, Friera AM, White K, Young C, Basco R, Lamba M, Wells F, Ankala R, Dowdle WE, Lin A, Egenberger K, Rukstalis JM, Millman JR, Connolly AJ, Deuse T, Schrepfer S (2023) Human hypoimmune primary pancreatic islets avoid rejection and autoimmunity and alleviate diabetes in allogeneic humanized mice. Sci Transl Med 15:eadg5794. https://doi.org/10.1126/scitranslmed.adg5794
Hu X, White K, Olroyd AG, DeJesus R, Dominguez AA, Dowdle WE, Friera AM, Young C, Wells F, Chu EY, Ito CE, Krishnapura H, Jain S, Ankala R, McGill TJ, Lin A, Egenberger K, Gagnon A, Michael Rukstalis J, Hogrebe NJ, Gattis C, Basco R, Millman JR, Kievit P, Davis MM, Lanier LL, Connolly AJ, Deuse T, Schrepfer S (2024) Hypoimmune induced pluripotent stem cells survive long term in fully immunocompetent, allogeneic rhesus macaques. Nat Biotechnol 42:413–423. https://doi.org/10.1038/s41587-023-01784-x
Bergström M, Yao M, Müller M, Korsgren O, von Zur-Mühlen B, Lundgren T (2021) Autologous regulatory T cells in clinical intraportal allogenic pancreatic islet transplantation. Transpl Int 34:2816–2823. https://doi.org/10.1111/tri.14163
Herold KC, Bundy BN, Long SA, Bluestone JA, DiMeglio LA, Dufort MJ, Gitelman SE, Gottlieb PA, Krischer JP, Linsley PS, Marks JB, Moore W, Moran A, Rodriguez H, Russell WE, Schatz D, Skyler JS, Tsalikian E, Wherrett DK, Ziegler AG, Greenbaum CJ (2019) An anti-CD3 antibody, Teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 381:603–613. https://doi.org/10.1056/NEJMoa1902226
Merani S, Toso C, Emamaullee J, Shapiro AM (2008) Optimal implantation site for pancreatic islet transplantation. Br J Surg 95:1449–1461. https://doi.org/10.1002/bjs.6391
Addison P, Fatakhova K, Rodriguez Rilo HL (2020) Considerations for an alternative site of islet cell transplantation. J Diabetes Sci Technol 14:338–344. https://doi.org/10.1177/1932296819868495
Inagaki A, Imura T, Nakamura Y, Ohashi K, Goto M (2021) The liver surface is an attractive transplant site for pancreatic islet transplantation. J Clin Med. https://doi.org/10.3390/jcm10040724
Pepper AR, Gala-Lopez B, Pawlick R, Merani S, Kin T, Shapiro AM (2015) A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nat Biotechnol 33:518–523. https://doi.org/10.1038/nbt.3211
Kinney SM, Ortaleza K, Vlahos AE, Sefton MV (2022) Degradable methacrylic acid-based synthetic hydrogel for subcutaneous islet transplantation. Biomaterials 281:121342. https://doi.org/10.1016/j.biomaterials.2021.121342
Pellicciaro M, Vella I, Lanzoni G, Tisone G, Ricordi C (2017) The greater omentum as a site for pancreatic islet transplantation. CellR4 Repair Replace Regen Reprogram 5:e2410
Damyar K, Farahmand V, Whaley D, Alexander M, Lakey JRT (2021) An overview of current advancements in pancreatic islet transplantation into the omentum. Islets 13:115–120. https://doi.org/10.1080/19382014.2021.1954459
Yasunami Y, Lacy PE, Finke EH (1983) A new site for islet transplantation–a peritoneal-omental pouch. Transplantation 36:181–182. https://doi.org/10.1097/00007890-198308000-00014
Ao ZL, Matayoshi K, Lakey JRT, Rajotte RV, Warnock GL (1993) Survival and function of purified islets in the omental pouch site of outbred dogs. Transplantation 56:524–529. https://doi.org/10.1097/00007890-199309000-00007
Deng H, Zhang A, Pang DRR, Xi Y, Yang Z, Matheson R, Li G, Luo H, Lee KM, Fu Q, Zou Z, Chen T, Wang Z, Rosales IA, Peters CW, Yang J, Coronel MM, Yolcu ES, Shirwan H, García AJ, Markmann JF, Lei J (2023) Bioengineered omental transplant site promotes pancreatic islet allografts survival in non-human primates. Cell Rep Med 4:100959. https://doi.org/10.1016/j.xcrm.2023.100959
Schwarzer A, Talbot SR, Selich A, Morgan M, Schott JW, Dittrich-Breiholz O, Bastone AL, Weigel B, Ha TC, Dziadek V, Gijsbers R, Thrasher AJ, Staal FJT, Gaspar HB, Modlich U, Schambach A, Rothe M (2021) Predicting genotoxicity of viral vectors for stem cell gene therapy using gene expression-based machine learning. Mol Ther 29:3383–3397. https://doi.org/10.1016/j.ymthe.2021.06.017
Hentze H, Soong PL, Wang ST, Phillips BW, Putti TC, Dunn NR (2009) Teratoma formation by human embryonic stem cells: Evaluation of essential parameters for future safety studies. Stem Cell Res 2:198–210. https://doi.org/10.1016/j.scr.2009.02.002
Pellegrini S, Zamarian V, Sordi V (2022) Strategies to improve the safety of iPSC-derived β cells for β cell replacement in diabetes. Trans Int. https://doi.org/10.3389/ti.2022.10575
Jiang W, Sui X, Zhang DH, Liu M, Ding MX, Shi Y, Deng HK (2011) CD24: a novel surface marker for PDX1-positive pancreatic progenitors derived from human embryonic stem cells. Stem Cells 29:609–617. https://doi.org/10.1002/stem.608
Ameri J, Borup R, Prawiro C, Ramond C, Schachter KA, Scharfmann R, Semb H (2017) Efficient generation of glucose-responsive beta cells from isolated GP2 human pancreatic progenitors. Cell Rep 19:36–49. https://doi.org/10.1016/j.celrep.2017.03.032
Cogger KF, Sinha A, Sarangi F, McGaugh EC, Saunders D, Dorrell C, Mejia-Guerrero S, Aghazadeh Y, Rourke JL, Screaton RA, Grompe M, Streeter PR, Powers AC, Brissova M, Kislinger T, Nostro MC (2017) Glycoprotein 2 is a specific cell surface marker of human pancreatic progenitors. Nat Commun. https://doi.org/10.1038/s41467-017-00561-0
Mahaddalkar PU, Scheibner K, Pfluger S, Ansarullah SM, Beckenbauer J, Irmler M, Beckers J, Knöbel S, Lickert H (2020) Generation of pancreatic β cells from CD177 anterior definitive endoderm. Nat Biotechnol 38:1061. https://doi.org/10.1038/s41587-020-0492-5
Salinno C, Büttner M, Cota P, Tritschler S, Tarquis-Medina M, Bastidas-Ponce A, Scheibner K, Burtscher I, Böttcher A, Theis FJ, Bakhti M, Lickert H (2021) CD81 marks immature and dedifferentiated pancreatic β-cells. Mol Metab. https://doi.org/10.1016/j.molmet.2021.101188
Tang C, Lee AS, Volkmer JP, Sahoo D, Nag D, Mosley AR, Inlay MA, Ardehali R, Chavez SL, Pera RR, Behr B, Wu JC, Weissman IL, Drukker M (2011) An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat Biotechnol 29:829-U86. https://doi.org/10.1038/nbt.1947
Pellegrini S, Zamarian V, Sordi V (2022) Strategies to improve the safety of iPSC-derived beta cells for beta cell replacement in diabetes. Transpl Int 35:10575. https://doi.org/10.3389/ti.2022.10575
Sheikh S, Ernst D, Keating A (2021) Prodrugs and prodrug-activated systems in gene therapy. Mol Ther 29:1716–1728. https://doi.org/10.1016/j.ymthe.2021.04.006
Nagashima T, Shimizu K, Matsumoto R, Honda H (2018) Selective elimination of human induced pluripotent stem cells using medium with high concentration of L-alanine. Sci Rep 8:12427. https://doi.org/10.1038/s41598-018-30936-2
Qadir MMF, Alvarez-Cubela S, Belle K, Sapir T, Messaggio F, Johnson KB, Umland O, Hardin D, Klein D, Perez-Alvarez I, Sadiq F, Alcazar O, Inverardi LA, Ricordi C, Buchwald P, Fraker CA, Pastori RL, Dominguez-Bendala J (2019) A double fail-safe approach to prevent tumorigenesis and select pancreatic beta cells from human embryonic stem cells. Stem Cell Reports 12:611–623. https://doi.org/10.1016/j.stemcr.2019.01.012
Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, Straathof K, Liu E, Durett AG, Grilley B, Liu H, Cruz CR, Savoldo B, Gee AP, Schindler J, Krance RA, Heslop HE, Spencer DM, Rooney CM, Brenner MK (2011) Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med 365:1673–1683. https://doi.org/10.1056/NEJMoa1106152
Liu Y, Yang Y, Suo Y, Li C, Chen M, Zheng S, Li H, Tang C, Fan N, Lan T, Zhou J, Li Y, Wang J, Chen H, Zou Q, Lai L (2022) Inducible caspase-9 suicide gene under control of endogenous oct4 to safeguard mouse and human pluripotent stem cell therapy. Mol Ther Methods Clin Dev 24:332–341. https://doi.org/10.1016/j.omtm.2022.01.014
Yagyu S, Hoyos V, Del Bufalo F, Brenner MK (2015) An suicide gene to improve the safety of therapy using human induced pluripotent stem cells. Mol Ther 23:1475–1485. https://doi.org/10.1038/mt.2015.100
Shi ZD, Tchao J, Wu L, Carman AJ (2020) Precision installation of a highly efficient suicide gene safety switch in human induced pluripotent stem cells. Stem Cells Transl Med 9:1378–1388. https://doi.org/10.1002/sctm.20-0007
Funding
This study was supported by the National Natural Science Foundation of China (No. 82270846).
Author information
Authors and Affiliations
Contributions
XRF, HMZ, SSY, DXC, YTW, XCQ and ZGS conceptualization. XRF and ZGS: writing original draft and editing. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, X., Zhang, H., Yang, S. et al. From stem cells to pancreatic β-cells: strategies, applications, and potential treatments for diabetes. Mol Cell Biochem (2024). https://doi.org/10.1007/s11010-024-04999-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-024-04999-x