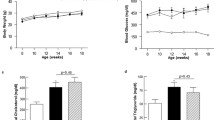
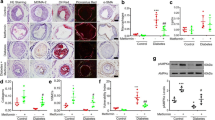
Abstract
Diabetes is a major risk factor for cardiovascular disease. However, the exact mechanism by which diabetes contributes to vascular damage is not fully understood. The aim of this study was to investigate the role of SUMO-1 mediated SERCA2a SUMOylation in the development of atherosclerotic vascular injury associated with diabetes mellitus. ApoE−/− mice were treated with streptozotocin (STZ) injection combined with high-fat feeding to simulate diabetic atherosclerosis and vascular injury. Human aortic vascular smooth muscle cells (HAVSMCs) were treated with high glucose (HG, 33.3 mM) and palmitic acid (PA, 200 µM) for 24 h to mimic a model of diabetes-induced vascular injury in vitro. Aortic vascular function, phenotypic conversion, migration, proliferation, intracellular Ca2+ concentration, the levels of small ubiquitin-like modifier type 1 (SUMO1), SERCA2a and SUMOylated SERCA2a were detected. Diabetes-induced atherosclerotic mice presented obvious atherosclerotic plaques and vascular injury, companied by significantly lower levels of SUMO1 and SERCA2a in aorta. HG and PA treatment in HAVSMCs reduced the expressions of SUMO1, SERCA2a and SUMOylated SERCA2a, facilitated the HAVSMCs phenotypic transformation, proliferation and migration, attenuated the Ca2+ transport, and increased the resting intracellular Ca2+ concentration. We also confirmed that SUMO1 directly bound to SERCA2a in HAVSMCs. Overexpression of SUMO1 restored the function and phenotypic contractile ability of HAVSMCs by upregulating SERCA2a SUMOylation, thereby alleviating HG and PA-induced vascular injury. These observations suggest an essential role of SUMO1 to protect diabetes-induced atherosclerosis and aortic vascular injury by the regulation of SERCA2a-SUMOylation and calcium homeostasis.
Graphic abstract






Similar content being viewed by others
Data availability
The data and materials of the current study are available from the corresponding author upon reasonable request.
Abbreviations
- CVDs :
-
Cardiovascular diseases
- CAD :
-
Coronary artery disease
- CHD :
-
Coronary heart disease
- STZ :
-
Streptozotocin
- HAVSMCs :
-
Human aortic vascular smooth muscle cells
- HG :
-
High glucose
- PA :
-
Palmitic acid
- BSA :
-
Bovine serum albumin
- HFD :
-
High-fat diet
- IP :
-
Immunoprecipitation
- IF :
-
Immunofluorescence
- SERCA2a :
-
Sarcoplasmic/endoplasmic reticulum calcium ATPase 2a
- SUMO :
-
Small ubiquitin-like modifier
- eNOS :
-
Endothelial nitric oxide synthase
- RUNX2 :
-
Runt-related transcription factor 2
- αSMA :
-
α-Smooth muscle actin
- SM22α :
-
Smooth muscle 22α
- RyR2 :
-
Ryanodine receptor 2
References
Zimmet P, Alberti KG, Magliano DJ, Bennett PH (2016) Diabetes mellitus statistics on prevalence and mortality: facts and fallacies. Nat Rev Endocrinol 12:616–622. https://doi.org/10.1038/nrendo.2016.105
Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE (2017) IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 128:40–50. https://doi.org/10.1016/j.diabres.2017.03.024
Henning RJ (2018) Type-2 diabetes mellitus and cardiovascular disease. Future Cardiol 14:491–509. https://doi.org/10.2217/fca-2018-0045
Paneni F, Beckman JA, Creager MA, Cosentino F (2013) Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J 34:2436–2443. https://doi.org/10.1093/eurheartj/eht149
Zhu Y, Xian X, Wang Z, Bi Y, Chen Q, Han X, Tang D, Chen R (2018) Research Progress on the relationship between atherosclerosis and inflammation. Biomolecules. https://doi.org/10.3390/biom8030080
Haas AV, McDonnell ME (2018) Pathogenesis of Cardiovascular Disease in Diabetes. Endocrinol Metab Clin North Am 47:51–63. https://doi.org/10.1016/j.ecl.2017.10.010
DEMIR ACARTÜRKE, M., KANADASI M (1999) Aortic atherosclerosis is a marker for significant coronary artery disease. Jpn Heart J 40(6):775–781. https://doi.org/10.1536/jhj.40.775
Basatemur GL, Jorgensen HF, Clarke MCH, Bennett MR, Mallat Z (2019) Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol 16:727–744. https://doi.org/10.1038/s41569-019-0227-9
Trion A, van der Laarse A (2004) Vascular smooth muscle cells and calcification in atherosclerosis. Am Heart J 147:808–814. https://doi.org/10.1016/j.ahj.2003.10.047
House SJ, Potier M, Bisaillon J, Singer HA, Trebak M (2008) The non-excitable smooth muscle: calcium signaling and phenotypic switching during vascular disease. Pflugers Arch 456:769–785. https://doi.org/10.1007/s00424-008-0491-8
Lipskaia L, Keuylian Z, Blirando K, Mougenot N, Jacquet A, Rouxel C, Sghairi H, Elaib Z, Blaise R, Adnot S, Hajjar RJ, Chemaly ER, Limon I, Bobe R (2014) Expression of sarco (endo) plasmic reticulum calcium ATPase (SERCA) system in normal mouse cardiovascular tissues, heart failure and atherosclerosis. Biochim Biophys Acta 1843:2705–2718. https://doi.org/10.1016/j.bbamcr.2014.08.002
Dally S, Corvazier E, Bredoux R, Bobe R, Enouf J (2010) Multiple and diverse coexpression, location, and regulation of additional SERCA2 and SERCA3 isoforms in nonfailing and failing human heart. J Mol Cell Cardiol 48:633–644. https://doi.org/10.1016/j.yjmcc.2009.11.012
Hadri L, Bobe R, Kawase Y, Ladage D, Ishikawa K, Atassi F, Lebeche D, Kranias EG, Leopold JA, Lompre AM, Lipskaia L, Hajjar RJ (2010) SERCA2a gene transfer enhances eNOS expression and activity in endothelial cells. Mol Ther 18:1284–1292. https://doi.org/10.1038/mt.2010.77
Sikkel MB, Hayward C, MacLeod KT, Harding SE, Lyon AR (2014) SERCA2a gene therapy in heart failure: an anti-arrhythmic positive inotrope. Br J Pharmacol 171:38–54. https://doi.org/10.1111/bph.12472
Hui HP, Li XY, Liu XH, Sun S, Lu XC, Liu T, Yang W (2006) Adeno-associated viral gene transfer of SERCA2a improves heart function in chronic congestive heart failure rats. Zhonghua Xin xue guan bing za zhi 34:357–362
Bobe R, Hadri L, Lopez JJ, Sassi Y, Atassi F, Karakikes I, Liang L, Limon I, Lompre AM, Hatem SN, Hajjar RJ, Lipskaia L (2011) SERCA2a controls the mode of agonist-induced intracellular Ca2 + signal, transcription factor NFAT and proliferation in human vascular smooth muscle cells. J Mol Cell Cardiol 50:621–633. https://doi.org/10.1016/j.yjmcc.2010.12.016
Sakata S, Lebeche D, Sakata Y, Sakata N, Chemaly ER, Liang L, Nakajima-Takenaka C, Tsuji T, Konishi N, del Monte F, Hajjar RJ, Takaki M (2007) Transcoronary gene transfer of SERCA2a increases coronary blood flow and decreases cardiomyocyte size in a type 2 diabetic rat model. Am J Physiol Heart Circ Physiol 292:H1204–H1207. https://doi.org/10.1152/ajpheart.00892.2006
Lipskaia L, del Monte F, Capiod T, Yacoubi S, Hadri L, Hours M, Hajjar RJ, Lompre AM (2005) Sarco/endoplasmic reticulum Ca2+-ATPase gene transfer reduces vascular smooth muscle cell proliferation and neointima formation in the rat. Circ Res 97:488–495. https://doi.org/10.1161/01.RES.0000180663.42594.aa
Zhihao L, Jingyu N, Lan L, Michael S, Rui G, Xiyun B, Xiaozhi L, Guanwei F (2020) SERCA2a: a key protein in the ca(2+) cycle of the heart failure. Heart Fail Rev 25:523–535. https://doi.org/10.1007/s10741-019-09873-3
Beltrao P, Bork P, Krogan NJ, van Noort V (2013) Evolution and functional cross-talk of protein post-translational modifications. Mol Syst Biol 9:714. https://doi.org/10.1002/msb.201304521
Tatham MH, Jaffray E, Vaughan OA, Desterro JM, Botting CH, Naismith JH, Hay RT (2001) Polymeric chains of SUMO-2 and SUMO-3 are conjugated to protein substrates by SAE1/SAE2 and Ubc9. J Biol Chem 276:35368–35374. https://doi.org/10.1074/jbc.M104214200
Yeh ET (2009) SUMOylation and De-SUMOylation: wrestling with life’s processes. J Biol Chem 284:8223–8227. https://doi.org/10.1074/jbc.R800050200
Kho C, Lee A, Jeong D, Oh JG, Chaanine AH, Kizana E, Park WJ, Hajjar RJ (2011) SUMO1-dependent modulation of SERCA2a in heart failure. Nature 477:601–605. https://doi.org/10.1038/nature10407
Lee A, Jeong D, Mitsuyama S, Oh JG, Liang L, Ikeda Y, Sadoshima J, Hajjar RJ, Kho C (2014) The role of SUMO-1 in cardiac oxidative stress and hypertrophy. Antioxid Redox Signal 21:1986–2001. https://doi.org/10.1089/ars.2014.5983
Liu YZ, Xiao X, Hu CT, Dai Y, Qu SL, Huang L, Zhang C (2020) SUMOylation in atherosclerosis. Clin Chim Acta 508:228–233. https://doi.org/10.1016/j.cca.2020.05.033
Dehnavi S, Sadeghi M, Penson PE, Banach M, Jamialahmadi T, Sahebkar A (2019) The role of protein SUMOylation in the pathogenesis of atherosclerosis. J Clin Med. https://doi.org/10.3390/jcm8111856
Leguina-Ruzzi A, Ortiz R, Velarde V (2018) The streptozotocin-high fat diet induced diabetic mouse model exhibits severe skin damage and alterations in local lipid mediators. Biomed J 41:328–332. https://doi.org/10.1016/j.bj.2018.08.005
Clee SM, Attie AD (2007) The genetic landscape of type 2 diabetes in mice. Endocr Rev 28:48–83. https://doi.org/10.1210/er.2006-0035
Si R, Zhang Q, Tsuji-Hosokawa A, Watanabe M, Willson C, Lai N, Wang J, Dai AZ, Scott BT, Dillmann WH, Yuan JXJ, Makino A (2020) Overexpression of p53 due to excess protein O-GlcNAcylation is associated with coronary microvascular disease in type 2 diabetes. Cardiovascular Res 116:1186–1198. https://doi.org/10.1093/cvr/cvz216
Lu L, Ma J, Sun M, Wang X, Gao E, Lu L, Ren J, Yang L, Yang J (2020) Melatonin ameliorates MI-Induced Cardiac Remodeling and apoptosis through a JNK/p53-Dependent mechanism in diabetes Mellitus. Oxid Med Cell Longev 2020:1535201. https://doi.org/10.1155/2020/1535201
Mathew AV, Zeng L, Atkins KB, Sadri KN, Byun J, Fujiwara H, Reddy P, Pennathur S (2021) Deletion of bone marrow myeloperoxidase attenuates chronic kidney disease accelerated atherosclerosis. J Biol Chem 296:100120. https://doi.org/10.1074/jbc.RA120.014095
Feng M, Xu D, Wang L (2018) miR-26a inhibits atherosclerosis progression by targeting TRPC3. Cell Biosci 8:4. https://doi.org/10.1186/s13578-018-0203-9
Reddy MA, Das S, Zhuo C, Jin W, Wang M, Lanting L, Natarajan R (2016) Regulation of vascular smooth muscle cell dysfunction under Diabetic conditions by miR-504. Arterioscler Thromb Vasc Biol 36:864–873. https://doi.org/10.1161/ATVBAHA.115.306770
Qiu C, Wang Y, Zhao H, Qin L, Shi Y, Zhu X, Song L, Zhou X, Chen J, Zhou H, Zhang H, Tellides G, Min W, Yu L (2017) The critical role of SENP1-mediated GATA2 deSUMOylation in promoting endothelial activation in graft arteriosclerosis. Nat Commun 8:15426. https://doi.org/10.1038/ncomms15426
Li Y, Cheng Q, Gao J, Chen Z, Guo J, Li Z, Tian L, Zhang C, Li Y, Zheng J, Li Z, Zhu J (2022) WWP1 upregulation predicts poor prognosis and promotes tumor progression by regulating ubiquitination of NDFIP1 in intrahepatic cholangiocarcinoma. Cell Death Discov 8:107. https://doi.org/10.1038/s41420-022-00882-0
National Research Council, et al. (1989) Diet and health: implications for reducing chronic disease risk, Washington (DC) .
Menini S, Iacobini C, Ricci C, Blasetti Fantauzzi C, Pugliese G (2015) Protection from diabetes-induced atherosclerosis and renal disease by D-carnosine-octylester: effects of early vs late inhibition of advanced glycation end-products in apoe-null mice. Diabetologia 58:845–853. https://doi.org/10.1007/s00125-014-3467-6
Plump AS, Smith JD, Hayek T, Aalto-Setälä K, Walsh A, Verstuyft JG, Breslow JL (1992) Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES Cells. Cell 71:343–53
Wang J, Zhao P, Gao Y, Zhang F, Yuan X, Jiao Y, Gong K (2019) The effects of Anti-IL-23p19 therapy on Atherosclerosis Development in ApoE(-/-) mice. J Interferon Cytokine Res 39:564–571. https://doi.org/10.1089/jir.2019.0050
Ma S, Chen J, Feng J, Zhang R, Fan M, Han D, Li X, Li C, Ren J, Wang Y, Cao F (2018) Melatonin ameliorates the progression of atherosclerosis via Mitophagy activation and NLRP3 inflammasome inhibition. Oxid Med Cell Longev 2018:9286458. https://doi.org/10.1155/2018/9286458
Gaetani S, Fan A, Wu X, Wu H, Li L, Huang R, Zhu Y, Qiu Y, Fu J, Ren J, Zhu C (2014) Atheroprotective Effect of Oleoylethanolamide (OEA) targeting oxidized LDL. PLoS ONE. https://doi.org/10.1371/journal.pone.0085337
Gimbrone MA Jr., Garcia-Cardena G (2016) Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res 118:620–636. https://doi.org/10.1161/CIRCRESAHA.115.306301
Grootaert MOJ, Moulis M, Roth L, Martinet W, Vindis C, Bennett MR, De Meyer GRY (2018) Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc Res 114:622–634. https://doi.org/10.1093/cvr/cvy007
Jebari-Benslaiman S, Galicia-Garcia U, Larrea-Sebal A, Olaetxea JR, Alloza I, Vandenbroeck K, Benito-Vicente A, Martin C (2022) Pathophysiology of atherosclerosis. Int J Mol Sci. https://doi.org/10.3390/ijms23063346
Frismantiene A, Philippova M, Erne P, Resink TJ (2018) Smooth muscle cell-driven vascular diseases and molecular mechanisms of VSMC plasticity. Cell Signal 52:48–64. https://doi.org/10.1016/j.cellsig.2018.08.019
Zhang F, Guo X, Xia Y, Mao L (2021) An update on the phenotypic switching of vascular smooth muscle cells in the pathogenesis of atherosclerosis. Cell Mol Life Sci 79:6. https://doi.org/10.1007/s00018-021-04079-z
Burger F, Baptista D, Roth A, da Silva RF, Montecucco F, Mach F, Brandt KJ, Miteva K (2021) NLRP3 inflammasome activation controls vascular smooth muscle cells phenotypic switch in atherosclerosis. Int J Mol Sci. https://doi.org/10.3390/ijms23010340
Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, Orekhov AN (2020) The diabetes Mellitus-Atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci. https://doi.org/10.3390/ijms21051835
Iwamoto M, Kubota T, Sakurai Y, Wada N, Shioda S, Yamauchi T, Kadowaki T, Kubota N (2022) The sodium-glucose co-transporter 2 inhibitor tofogliflozin suppresses atherosclerosis through glucose lowering in ApoE-deficient mice with streptozotocin-induced diabetes. Pharmacol Res Perspect 10:e00971. https://doi.org/10.1002/prp2.971
Matchkov VV, Kudryavtseva O, Aalkjaer C (2012) Intracellular ca(2)(+) signalling and phenotype of vascular smooth muscle cells. Basic Clin Pharmacol Toxicol 110:42–48. https://doi.org/10.1111/j.1742-7843.2011.00818.x
Magnier-Gaubil C, Herbert JM, Quarck R, Papp B, Corvazier E, Wuytack F, Levy-Toledano S, Enouf J (1996) Smooth muscle cell cycle and proliferation. Relationship between calcium influx and sarco-endoplasmic reticulum Ca2 + ATPase regulation. J Biol Chem 271:27788–27794. https://doi.org/10.1074/jbc.271.44.27788
Funding
This work was supported by the National Natural Science Foundation of China (#32171181 and #31900534), the Natural Science Foundation of Hebei Province (#C2019201349), the Hundred Talents Funding Program of Hebei Province (#E2019050010), the Interdisciplinary Research Program of Natural Science of Hebei University (#DXK202105) and the Advanced Talents Incubation Program of the Hebei University (#801260201282).
Author information
Authors and Affiliations
Contributions
Conceptualization was performed by RG. Study design and methodology were performed by JL and RG. Result validation and interpretation were performed by JL, SX and RG. Data analysis and figures preparation were performed by JL, SX, BG and MY. Writing—original draft preparation were performed by JL and SX. Writing—review and editing were performed by LZ and RG. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All experimental procedures and methods were approved by the Hebei University Animal Care and Use Committee (Approval No. IACUC-2021XG032).
Consent to publish
We have obtained consent to publish this paper from all the participants of this research.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, J., Xu, S., Gao, B. et al. Protective effect of SERCA2a-SUMOylation by SUMO-1 on diabetes-induced atherosclerosis and aortic vascular injury. Mol Cell Biochem (2024). https://doi.org/10.1007/s11010-024-04953-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-024-04953-x