Abstract
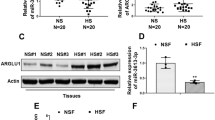
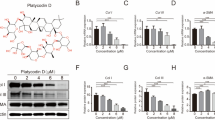
Hypertrophic scar (HS) formation is a cutaneous fibroproliferative disease that occurs after skin injuries and results in severe functional and esthetic disability. To date, few drugs have shown satisfactory outcomes for the treatment of HS formation. Transforming growth factor-beta (TGF-β)/Notch interaction via small mothers against decapentaplegic 3 (Smad3) could facilitate HS formation; therefore, targeting TGF-β/ Notch interaction via Smad3 is a potential therapeutic strategy to attenuate HS formation. In addition, optic atrophy 1 (OPA1)-mediated mitochondrial fusion contributes to fibroblast proliferation, and TGF-β/Smad3 axis and the Notch1 pathway facilitate OPA1-mediated mitochondrial fusion. Thus, the aim of this study was to investigate whether drugs targeting TGF-β/Notch interaction via Smad3 suppressed fibroblast proliferation to attenuate HS formation through OPA1-mediated mitochondrial fusion. We found that the TGF-β pathway, Notch pathway, and TGF-β/Notch interaction via Smad3 were inhibited by pirfenidone, the gamma- secretase inhibitor DAPT, and SIS3 in human keloid fibroblasts (HKF) and an HS rat model, respectively. Protein interaction was detected by co-immunoprecipitation, and mitochondrial morphology was determined by electron microscopy. Our results indicated that pirfenidone, DAPT, and SIS3 suppressed the proliferation of HKFs and attenuated HS formation in the HS rat model by inhibiting TGF-β/Notch interaction via Smad3. Moreover, pirfenidone, DAPT, and SIS3 hindered OPA1-mediated mitochondrial fusion through inhibiting TGF-β/Notch interaction, thereby suppressing the proliferation of HS fibroblasts and HS formation. In summary, these findings investigating the effects of drugs targeting TGF-β/Notch interaction on HS formation might lead to novel drugs for the treatment of HS formation.







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Gao C, Lu C, Jian Z, Zhang T, Chen Z, Zhu Q et al (2021) 3D bioprinting for fabricating artificial skin tissue. Colloids Surf B 208:112041. https://doi.org/10.1016/j.colsurfb.2021.112041
Zhang T, Wang XF, Wang ZC, Lou D, Fang QQ, Hu YY et al (2020) Current potential therapeutic strategies targeting the TGF-beta/Smad signaling pathway to attenuate keloid and hypertrophic scar formation. Biomed Pharmacother 129:110287. https://doi.org/10.1016/j.biopha.2020.110287
Lee HJ, Jang YJ (2018) Recent understandings of Biology, Prophylaxis and Treatment strategies for hypertrophic scars and keloids. Int J Mol Sci. https://doi.org/10.3390/ijms030711
He J, Fang B, Shan S, Xie Y, Wang C, Zhang Y et al (2021) Mechanical stretch promotes hypertrophic scar formation through mechanically activated cation channel Piezo1. Cell Death Dis 12:226. https://doi.org/10.1038/s41419-021-03481-6
Agarwal S, Sorkin M, Levi B (2017) Heterotopic ossification and hypertrophic scars. Clin Plast Surg 44:749–755. https://doi.org/10.1016/j.cps.2017.05.006
Chiang RS, Borovikova AA, King K, Banyard DA, Lalezari S, Toranto JD et al (2016) Current concepts related to hypertrophic scarring in burn injuries. Wound Repair Regen 24:466–477. https://doi.org/10.1111/wrr.12432
Ma K, Kwon SH, Padmanabhan J, Duscher D, Trotsyuk AA, Dong Y et al (2018) Controlled delivery of a focal adhesion kinase inhibitor results in Accelerated Wound Closure with decreased scar formation. J Invest Dermatol 138:2452–2460. https://doi.org/10.1016/j.jid.2018.04.034
Taheri A, Mansoori P, Al-Dabagh A, Feldman SR (2014) Are corticosteroids effective for prevention of scar formation after second-degree skin burn? J Dermatol Treat 25:360–362. https://doi.org/10.3109/09546634.2013.806768
Caliskan E, Gamsizkan M, Acikgoz G, Durmus M, Toklu S, Dogrul A et al (2016) Intralesional treatments for hypertrophic scars: comparison among corticosteroid, 5-fluorouracil and botulinum toxin in rabbit ear hypertrophic scar model. Eur Rev Med Pharmacol Sci 20:1603–1608
Berman B, Maderal A, Raphael B (2017) Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatol Surg 43(Suppl 1):S3–S18. https://doi.org/10.1097/DSS.0000000000000819
Ru Z, Hu Y, Huang S, Bai L, Zhang K, Li Y (2021) Bioflavonoid Galangin suppresses hypertrophic scar formation by the TGF-beta/Smad signaling pathway. Evid Based Complement Alternat Med 2021:2444839. https://doi.org/10.1155/2021/2444839
Wells AR, Leung KP (2020) Pirfenidone attenuates the profibrotic contractile phenotype of differentiated human dermal myofibroblasts. Biochem Biophys Res Commun 521:646–651. https://doi.org/10.1016/j.bbrc.2019.10.177
Medina JL, Sebastian EA, Fourcaudot AB, Dorati R, Leung KP (2019) Pirfenidone Ointment modulates the burn Wound Bed in C57BL/6 mice by suppressing inflammatory responses. Inflammation 42:45–53. https://doi.org/10.1007/s10753-018-0871-y
Armendariz-Borunda J, Lyra-Gonzalez I, Medina-Preciado D, Gonzalez-Garcia I, Martinez-Fong D, Miranda RA et al (2012) A controlled clinical trial with pirfenidone in the treatment of pathological skin scarring caused by Burns in pediatric patients. Ann Plast Surg 68:22–28. https://doi.org/10.1097/SAP.0b013e31821b6d08
Cantu-Cantu MZ, Lyra-Gonzalez I, Armendariz-Borunda J (2013) Coadjuvant treatment with Surgery and pirfenidone in severe facial trauma due to dog bite. J Craniofac Surg 24:675–678. https://doi.org/10.1097/SCS.0b013e31828609cb
He T, Bai X, Jing J, Liu Y, Wang H, Zhang W et al (2020) Notch signal deficiency alleviates hypertrophic scar formation after wound healing through the inhibition of inflammation. Arch Biochem Biophys 682:108286. https://doi.org/10.1016/j.abb.2020.108286
Li B, Gao C, Diao JS, Wang DL, Chu FF, Li Y et al (2016) Aberrant notch signalling contributes to hypertrophic scar formation by modulating the phenotype of keratinocytes. Exp Dermatol 25:137–142. https://doi.org/10.1111/exd.12897
Xia Z, Wang J, Yang S, Liu C, Qin S, Li W et al (2021) Emodin alleviates hypertrophic scar formation by suppressing macrophage polarization and inhibiting the notch and TGF-beta pathways in macrophages. Braz J Med Biol Res 54:e11184. https://doi.org/10.1590/1414-431X2021e11184
Conedera FM, Pousa AMQ, Mercader N, Tschopp M, Enzmann V (2021) The TGFbeta/Notch axis facilitates Muller cell-to-epithelial transition to ultimately form a chronic glial scar. Mol Neurodegener 16:69. https://doi.org/10.1186/s13024-021-00482-z
Kleele T, Rey T, Winter J, Zaganelli S, Mahecic D, Perreten Lambert H et al (2021) Distinct fission signatures predict mitochondrial degradation or biogenesis. Nature 593:435–439. https://doi.org/10.1038/s41586-021-03510-6
Yao CH, Wang R, Wang Y, Kung CP, Weber JD, Patti GJ (2019) Mitochondrial fusion supports increased oxidative phosphorylation during cell proliferation. Elife. https://doi.org/10.7554/eLife.41351
Chan DC (2020) Mitochondrial dynamics and its involvement in Disease. Annu Rev Pathol 15:235–259. https://doi.org/10.1146/annurev-pathmechdis-012419-032711
Giacomello M, Pyakurel A, Glytsou C, Scorrano L (2020) The cell biology of mitochondrial membrane dynamics. Nat Rev Mol Cell Biol 21:204–224. https://doi.org/10.1038/s41580-020-0210-7
Li M, Wang L, Wang Y, Zhang S, Zhou G, Lieshout R et al (2020) Mitochondrial fusion Via OPA1 and MFN1 supports liver tumor cell metabolism and growth. Cells. https://doi.org/10.3390/cells9010121
Baixauli F, Piletic K, Puleston DJ, Villa M, Field CS, Flachsmann LJ et al (2022) An LKB1-mitochondria axis controls T(H)17 effector function. Nature 610:555–561. https://doi.org/10.1038/s41586-022-05264-1
Silva Ramos E, Motori E, Bruser C, Kuhl I, Yeroslaviz A, Ruzzenente B et al (2019) Mitochondrial fusion is required for regulation of mitochondrial DNA replication. PLoS Genet 15:e1008085. https://doi.org/10.1371/journal.pgen.1008085
Zhao Y, Zhu J, Zhang N, Liu Q, Wang Y, Hu X et al (2020) GDF11 enhances therapeutic efficacy of mesenchymal stem cells for Myocardial Infarction via YME1L-mediated OPA1 processing. Stem Cells Transl Med 9:1257–1271. https://doi.org/10.1002/sctm.20-0005
Dubal D, Moghe P, Verma RK, Uttekar B, Rikhy R (2022) Mitochondrial fusion regulates proliferation and differentiation in the type II neuroblast lineage in Drosophila. PLOS Genet 18:e1010055. https://doi.org/10.1371/journal.pgen.1010055
Brown KA, Ham AJ, Clark CN, Meller N, Law BK, Chytil A et al (2008) Identification of novel Smad2 and Smad3 associated proteins in response to TGF-beta1. J Cell Biochem 105:596–611. https://doi.org/10.1002/jcb.21860
Ou LD, Zhang AJ, Li A, Tao SJ, Xu MM, Li Q et al (2019) Effect of human stromal vascular fraction gel on the treatment of patients with skin depressed scar and its mechanism. Zhonghua Shao Shang Za Zhi 35:859–865. https://doi.org/10.3760/cma.j.issn.1009-2587.2019.12.006
Lv Q, Wang J, Xu C, Huang X, Ruan Z, Dai Y (2020) Pirfenidone alleviates pulmonary fibrosis in vitro and in vivo through regulating Wnt/GSK-3beta/beta-catenin and TGF-beta1/Smad2/3 signaling pathways. Mol Med 26:49. https://doi.org/10.1186/s10020-020-00173-3
Li G, Ren J, Hu Q, Deng Y, Chen G, Guo K et al (2016) Oral pirfenidone protects against fibrosis by inhibiting fibroblast proliferation and TGF-beta signaling in a murine Colitis model. Biochem Pharmacol 117:57–67. https://doi.org/10.1016/j.bcp.2016.08.002
Dees C, Tomcik M, Zerr P, Akhmetshina A, Horn A, Palumbo K et al (2011) Notch signalling regulates fibroblast activation and collagen release in systemic sclerosis. Ann Rheum Dis 70:1304–1310. https://doi.org/10.1136/ard.2010.134742
Sassoli C, Chellini F, Pini A, Tani A, Nistri S, Nosi D et al (2013) Relaxin prevents cardiac fibroblast-myofibroblast transition via notch-1-mediated inhibition of TGF-beta/Smad3 signaling. PLOS ONE 8:e63896. https://doi.org/10.1371/journal.pone.0063896
Fan YH, Dong H, Pan Q, Cao YJ, Li H, Wang HC (2011) Notch signaling may negatively regulate neonatal rat cardiac fibroblast-myofibroblast transformation. Physiol Res 60:739–748. https://doi.org/10.33549/physiolres.932149
Shah MH, Chan EC, Van Bergen NJ, Pandav SS, Ng S, Crowston JG et al (2020) Nox4 facilitates TGFbeta1-induced fibrotic response in human tenon’s fibroblasts and promotes wound collagen accumulation in murine model of glaucoma filtration surgery. Antioxidants 9:1126. https://doi.org/10.3390/antiox9111126
Du Y, Zhu P, Wang X, Mu M, Li H, Gao Y et al (2020) Pirfenidone alleviates lipopolysaccharide-induced lung injury by accentuating BAP31 regulation of ER stress and mitochondrial injury. J Autoimmun 112:102464. https://doi.org/10.1016/j.jaut.2020.102464
Plataki M, Cho SJ, Harris RM, Huang HR, Yun HS, Schiffer KT et al (2019) Mitochondrial dysfunction in aged macrophages and lung during primary Streptococcus pneumoniae Infection is improved with Pirfenidone. Sci Rep 9:971. https://doi.org/10.1038/s41598-018-37438-1
Zhang M, Wu P, Li M, Guo Y, Tian T, Liao X et al (2021) Inhibition of Notch1 signaling reduces hepatocyte injury in nonalcoholic fatty Liver Disease via autophagy. Biochem Biophys Res Commun 547:131–138. https://doi.org/10.1016/j.bbrc.2021.02.039
Jiang M, Fan J, Qu X, Li S, Nilsson SK, Sun YBY et al (2019) Combined blockade of Smad3 and JNK pathways Ameliorates Progressive Fibrosis in Folic Acid Nephropathy. Front Pharmacol 10:880. https://doi.org/10.3389/fphar.2019.00880
Shen X, Hu PP, Liberati NT, Datto MB, Frederick JP, Wang XF (1998) TGF-beta-induced phosphorylation of Smad3 regulates its interaction with coactivator p300/CREB-binding protein. Mol Biol Cell 9:3309–3319. https://doi.org/10.1091/mbc.9.12.3309
Thomas AL, Lind H, Hong A, Dokic D, Oppat K, Rosenthal E et al (2017) Inhibition of CDK-mediated Smad3 phosphorylation reduces the Pin1-Smad3 interaction and aggressiveness of triple negative Breast cancer cells. Cell Cycle 16:1453–1464. https://doi.org/10.1080/15384101.2017.1338988
Charbonney E, Speight P, Masszi A, Nakano H, Kapus A (2011) beta-catenin and Smad3 regulate the activity and stability of myocardin-related transcription factor during epithelial-myofibroblast transition. Mol Biol Cell 22:4472–4485. https://doi.org/10.1091/mbc.E11-04-0335
Funding
This work was supported by Guangdong Provincial Medical Science and Technology Research Fund project (#B2023167).
Author information
Authors and Affiliations
Contributions
Guarantor of integrity of the entire study: DH; study concepts: DH; study design: DH; definition of intellectual content: XB; literature research: DH; clinical studies: JZ; experimental studies: JZ; data acquisition: DD; data analysis: DD; statistical analysis: DH; manuscript preparation: XB; manuscript editing: XD; manuscript review: DH.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have read the journal’s policy on disclosing potential conflicts of interest, and they all declare no personal or financial conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huo, D., Bi, Xy., Zeng, Jl. et al. Drugs targeting TGF-β/Notch interaction attenuate hypertrophic scar formation by optic atrophy 1-mediated mitochondrial fusion. Mol Cell Biochem (2023). https://doi.org/10.1007/s11010-023-04912-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-023-04912-y