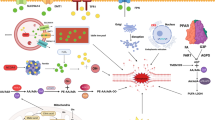
Abstract
Hepatocellular carcinoma (HCC) is the sixed most common malignant tumor in the world. The study for HCC is mired in the predicament confronted with the difficulty of early diagnosis and high drug resistance, the survival rate of patients with HCC being low. Ferroptosis, an iron-dependent cell death, has been discovered in recent years as a cell death means with tremendous potential to fight against cancer. The in-depth researches for iron metabolism, lipid peroxidation and dysregulation of antioxidant defense have brought about tangible progress in the firmament of ferroptosis with more and more results showing close connections between ferroptosis and HCC. The potential role of ferroptosis has been widely used in chemotherapy, immunotherapy, radiotherapy, and nanotherapy, with the development of various new drugs significantly improving the prognosis of patients. Based on the characteristics and mechanisms of ferroptosis, this article further focuses on the main signaling pathways and promising treatments of HCC, envisioning that existing problems in regard with ferroptosis and HCC could be grappled with in the foreseeable future.

Similar content being viewed by others
Data availability
Enquiries about data availability should be directed to the authors.
References
He P, Wan H, Wan J, Jiang H, Yang Y, Xie K et al (2022) Systemic therapies in hepatocellular carcinoma: existing and emerging biomarkers for treatment response. Front Oncol 12:1015527. https://doi.org/10.3389/fonc.2022.1015527
Zhou J, Li LU, Fang LI, Xie H, Yao W, Zhou X et al (2016) Quercetin reduces cyclin D1 activity and induces G1 phase arrest in HepG2 cells. Oncol Lett 12:516–522. https://doi.org/10.3892/ol.2016.4639
Pang BY, Leng Y, Wang X, Wang YQ, Jiang LH (2023) A meta-analysis and of clinical values of 11 blood biomarkers, such as AFP, DCP, and GP73 for diagnosis of hepatocellular carcinoma. Ann Med 55:42–61. https://doi.org/10.1080/07853890.2022.2153163
Huang Z, Xia H, Cui Y, Yam JWP, Xu Y (2023) Ferroptosis: from basic research to clinical therapeutics in hepatocellular carcinoma. J Clin Transl Hepatol 11:207–218. https://doi.org/10.14218/jcth.2022.00255
Bae C, Kim H, Kook YM, Lee C, Kim C, Yang C et al (2022) Induction of ferroptosis using functionalized iron-based nanoparticles for anti-cancer therapy. Mater Today Bio 17:100457. https://doi.org/10.1016/j.mtbio.2022.100457
Fuchs Y, Steller H (2011) Programmed cell death in animal development and disease. Cell 147:1640. https://doi.org/10.1016/j.cell.2011.10.033
Li L, Wang X, Xu H, Liu X, Xu K (2022) Perspectives and mechanisms for targeting ferroptosis in the treatment of hepatocellular carcinoma. Front Mol Biosci 9:947208. https://doi.org/10.3389/fmolb.2022.947208
Peng F, Liao M, Qin R, Zhu S, Peng C, Fu L et al (2022) Regulated cell death (RCD) in cancer: key pathways and targeted therapies. Signal Transduct Target Ther 7:286. https://doi.org/10.1038/s41392-022-01110-y
Pan F, Lin X, Hao L, Wang T, Song H, Wang R (2022) The critical role of ferroptosis in hepatocellular carcinoma. Front Cell Dev Biol 10:882571. https://doi.org/10.3389/fcell.2022.882571
Shannon AH, Ruff SM, Pawlik TM (2022) Expert insights on current treatments for hepatocellular carcinoma: clinical and molecular approaches and bottlenecks to progress. J Hepatocell Carcinoma 9:1247–1261. https://doi.org/10.2147/jhc.S383922
Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R (2021) Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov 20:101–124. https://doi.org/10.1038/s41573-020-0090-8
Gan B (2021) Mitochondrial regulation of ferroptosis. J Cell Biol 220:e202105043. https://doi.org/10.1083/jcb.202105043
You H, Wang L, Bu F, Meng H, Huang C, Fang G et al (2022) Ferroptosis: shedding light on mechanisms and therapeutic opportunities in liver diseases. Cells. https://doi.org/10.3390/cells11203301
Scindia PHDY, Leeds MDJ, Swaminathan MDS (2019) Iron homeostasis in healthy kidney and its role in acute kidney injury. Semin Nephrol 39:76–84. https://doi.org/10.1016/j.semnephrol.2018.10.006
Tang Z, Huang Z, Huang Y, Chen Y, Huang M, Liu H et al (2021) Ferroptosis: the silver lining of cancer therapy. Front Cell Dev Biol 9:765859. https://doi.org/10.3389/fcell.2021.765859
Hassannia B, Vandenabeele P, Vanden Berghe T (2019) Targeting ferroptosis to iron out cancer. Cancer Cell 35:830–849. https://doi.org/10.1016/j.ccell.2019.04.002
Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I et al (2017) ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol 13:91–98. https://doi.org/10.1038/nchembio.2239
Yang Y, Zhu T, Wang X, Xiong F, Hu Z, Qiao X et al (2022) ACSL3 and ACSL4, distinct roles in ferroptosis and cancers. Cancers 14:5896. https://doi.org/10.3390/cancers14235896
Koppula P, Zhang Y, Zhuang L, Gan B (2018) Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun 38:12. https://doi.org/10.1186/s40880-018-0288-x
Imai H, Matsuoka M, Kumagai T, Sakamoto T, Koumura T (2017) Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. Curr Top Microbiol Immunol 403:143–170. https://doi.org/10.1007/82_2016_508
Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC (2014) Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 509:105–109. https://doi.org/10.1038/nature13148
Kakhlon O, Cabantchik ZI (2002) The labile iron pool: characterization, measurement, and participation in cellular processes. Free Radic Biol Med 33:1037–1046. https://doi.org/10.1016/s0891-5849(02)01006-7
Liu Z, Lv X, Song E, Song Y (2020) Fostered Nrf2 expression antagonizes iron overload and glutathione depletion to promote resistance of neuron-like cells to ferroptosis. Toxicol Appl Pharmacol 407:115241. https://doi.org/10.1016/j.taap.2020.115241
Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR (2016) Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci USA 113:E4966–E4975. https://doi.org/10.1073/pnas.1603244113
Dodson M, Castro-Portuguez R, Zhang DD (2019) NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol 23:101107. https://doi.org/10.1016/j.redox.2019.101107
Mladěnka P, Macáková K, Kujovská Krčmová L, Javorská L, Mrštná K, Carazo A et al (2022) Vitamin K—sources, physiological role, kinetics, deficiency, detection, therapeutic use, and toxicity. Nutr Rev 80:677–698. https://doi.org/10.1093/nutrit/nuab061
Deng F, Sharma I, Dai Y, Yang M, Kanwar YS (2019) Myo-inositol oxygenase expression profile modulates pathogenic ferroptosis in the renal proximal tubule. J Clin Invest 129:5033–5049. https://doi.org/10.1172/jci129903
He F, Zhang P, Liu J, Wang R, Kaufman RJ, Yaden BC et al (2023) ATF4 suppresses hepatocarcinogenesis by inducing SLC7A11 (xCT) to block stress-related ferroptosis. J Hepatol 79:362–377. https://doi.org/10.1016/j.jhep.2023.03.016
Long S, Peng F, Song B, Wang L, Chen J, Shang B (2021) Heat shock protein beta 1 is a prognostic biomarker and correlated with immune infiltrates in hepatocellular carcinoma. Int J Gen Med 14:5483–5492. https://doi.org/10.2147/ijgm.S330608
Suttner DM, Dennery PA (1999) Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. Faseb J 13:1800–1809. https://doi.org/10.1096/fasebj.13.13.1800
Liu M, Kong XY, Yao Y, Wang XA, Yang W, Wu H et al (2022) The critical role and molecular mechanisms of ferroptosis in antioxidant systems: a narrative review. Ann Transl Med 10:368. https://doi.org/10.21037/atm-21-6942
Park E, Chung SW (2019) ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis 10:822. https://doi.org/10.1038/s41419-019-2064-5
Huang BW, Miyazawa M, Tsuji Y (2014) Distinct regulatory mechanisms of the human ferritin gene by hypoxia and hypoxia mimetic cobalt chloride at the transcriptional and post-transcriptional levels. Cell Signal 26:2702–2709. https://doi.org/10.1016/j.cellsig.2014.08.018
Wei X, Yi X, Zhu XH, Jiang DS (2020) Posttranslational modifications in ferroptosis. Oxid Med Cell Longev 2020:8832043. https://doi.org/10.1155/2020/8832043
Chen X, Kang R, Kroemer G, Tang D (2021) Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol 18:280–296
Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS et al (2017) Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 13:81–90. https://doi.org/10.1038/nchembio.2238
Yan HF, Zou T, Tuo QZ, Xu S, Li H, Belaidi AA et al (2021) Ferroptosis: mechanisms and links with diseases. Signal Transduct Targets 6:49. https://doi.org/10.1038/s41392-020-00428-9
Jiang X, Stockwell BR, Conrad M (2021) Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol 22:266–282. https://doi.org/10.1038/s41580-020-00324-8
Zou Y, Li H, Graham ET, Deik AA, Eaton JK, Wang W et al (2020) Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat Chem Biol 16:302–309. https://doi.org/10.1038/s41589-020-0472-6
Galadari S, Rahman A, Pallichankandy S, Thayyullathil F (2017) Reactive oxygen species and cancer paradox: to promote or to suppress? Free Radic Biol Med 104:144–164. https://doi.org/10.1016/j.freeradbiomed.2017.01.004
Capelletti MM, Manceau H, Puy H, Peoc’h K (2020) Ferroptosis in liver diseases: an overview. Int J Mol Sci 21:4908. https://doi.org/10.3390/ijms21144908
Lin W, Wang C, Liu G, Bi C, Wang X, Zhou Q et al (2020) SLC7A11/xCT in cancer: biological functions and therapeutic implications. Am J Cancer Res 10:3106–3126
Qu L, He X, Tang Q, Fan X, Liu J, Lin A (2022) Iron metabolism, ferroptosis, and lncRNA in cancer: knowns and unknowns. J Zhejiang Univ Sci B 23:844–862. https://doi.org/10.1631/jzus.B2200194
Tang D, Chen X, Kang R, Kroemer G (2021) Ferroptosis: molecular mechanisms and health implications. Cell Res 31:107–125. https://doi.org/10.1038/s41422-020-00441-1
Gao Y, Li Y, Cao H, Jia H, Wang D, Ren C et al (2022) Hypertoxic self-assembled peptide with dual functions of glutathione depletion and biosynthesis inhibition for selective tumor ferroptosis and pyroptosis. J Nanobiotechnol 20:390. https://doi.org/10.1186/s12951-022-01604-5
Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH et al (2019) The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575:688–692. https://doi.org/10.1038/s41586-019-1705-2
Pallotti F, Bergamini C, Lamperti C, Fato R (2021) The roles of coenzyme Q in disease: direct and indirect involvement in cellular functions. Int J Mol Sci 23:128. https://doi.org/10.3390/ijms23010128
Zhang Y, Huang X, Liu N, Liu M, Sun C, Qi B et al (2022) Discovering the potential value of coenzyme Q10 in oxidative stress: enlightenment from a synthesis of clinical evidence based on various population. Front Pharmacol 14:936233. https://doi.org/10.3389/fphar.2022.936233
Yuan J, Lv T, Yang J, Wu Z, Yan L, Yang J et al (2022) HDLBP-stabilized lncFAL inhibits ferroptosis vulnerability by diminishing Trim69-dependent FSP1 degradation in hepatocellular carcinoma. Redox Biol 58:102546. https://doi.org/10.1016/j.redox.2022.102546
Kraft VAN, Bezjian CT, Pfeiffer S, Ringelstetter L, Müller C, Zandkarimi F et al (2020) GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. Acs Cent Sci 6:41–53. https://doi.org/10.1021/acscentsci.9b01063
Su J, Bian C, Zheng Z, Wang H, Meng L, Xin Y et al (2022) Cooperation effects of radiation and ferroptosis on tumor suppression and radiation injury. Front Cell Dev Biol 10:951116. https://doi.org/10.3389/fcell.2022.951116
Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee H et al (2021) DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 593:586–590. https://doi.org/10.1038/s41586-021-03539-7
Tian Y, Zhang M, Fan M, Xu H, Wu S, Zou S et al (2022) A miRNA-mediated attenuation of hepatocarcinogenesis in both hepatocytes and Kupffer cells. Mol Ther Nucleic Acids 30:1–12. https://doi.org/10.1016/j.omtn.2022.08.036
Dai SM, Li FJ, Long HZ, Zhou ZW, Luo HY, Xu SG et al (2022) Relationship between miRNA and ferroptosis in tumors. Front Pharmacol 13:977062. https://doi.org/10.3389/fphar.2022.977062
Lu Y, Chan YT, Tan HY, Zhang C, Guo W, Xu Y et al (2022) Epigenetic regulation of ferroptosis via ETS1/miR-23a-3p/ACSL4 axis mediates sorafenib resistance in human hepatocellular carcinoma. J Exp Clin Cancer Res 41:3. https://doi.org/10.1186/s13046-021-02208-x
Hu Z, Li L, Li M, Zhang X, Zhang Y, Ran J et al (2023) miR-21–5p inhibits ferroptosis in hepatocellular carcinoma cells by regulating the AKT/mTOR signaling pathway through MELK. J Immunol Res 2023:8929525. https://doi.org/10.1155/2023/8929525
Yao Z, Luo J, Hu K, Lin J, Huang H, Wang Q et al (2017) ZKSCAN1 gene and its related circular RNA (circZKSCAN1) both inhibit hepatocellular carcinoma cell growth, migration, and invasion but through different signaling pathways. Mol Oncol 11:422–437. https://doi.org/10.1002/1878-0261.12045
Zuo YB, Zhang YF, Zhang R, Tian JW, Lv XB, Li R et al (2022) Ferroptosis in cancer progression: role of noncoding RNAs. Int J Biol Sci 18:1829–1843. https://doi.org/10.7150/ijbs.66917
Lyu N, Zeng Y, Kong Y, Chen Q, Deng H, Ou S et al (2021) Ferroptosis is involved in the progression of hepatocellular carcinoma through the circ0097009/miR-1261/SLC7A11 axis. Ann Transl Med 9:675. https://doi.org/10.21037/atm-21-997
Xu Q, Zhou L, Yang G, Meng F, Wan Y, Wang L et al (2020) CircIL4R facilitates the tumorigenesis and inhibits ferroptosis in hepatocellular carcinoma by regulating the miR-541–3p/GPX4 axis. Cell Biol Int 44:2344–2356. https://doi.org/10.1002/cbin.11444
Liu Z, Wang Q, Wang X, Xu Z, Wei X, Li J (2020) Circular RNA cIARS regulates ferroptosis in HCC cells through interacting with RNA binding protein ALKBH5. Cell Death Discov 6:72. https://doi.org/10.1038/s41420-020-00306-x
Zhai H, Zhong S, Wu R, Mo Z, Zheng S, Xue J et al (2023) Suppressing circIDE/miR-19b-3p/RBMS1 axis exhibits promoting-tumour activity through upregulating GPX4 to diminish ferroptosis in hepatocellular carcinoma. Epigenetics 18:2192438. https://doi.org/10.1080/15592294.2023.2192438
Liu Y, Li J (2023) Circular RNA 0016142 knockdown induces ferroptosis in hepatocellular carcinoma cells via modulation of the MicroRNA-188–3p/glutathione peroxidase 4 axis. Biochem Genet. https://doi.org/10.1007/s10528-023-10417-6
Fang Y, Zhang X, Huang H, Zeng Z (2023) The interplay between noncoding RNAs and drug resistance in hepatocellular carcinoma: the big impact of little things. J Transl Med 21:369. https://doi.org/10.1186/s12967-023-04238-9
Qi W, Li Z, Xia L, Dai J, Zhang Q, Wu C et al (2019) LncRNA GABPB1-AS1 and GABPB1 regulate oxidative stress during erastin-induced ferroptosis in HepG2 hepatocellular carcinoma cells. Sci Rep 9:16185. https://doi.org/10.1038/s41598-019-52837-8
He GN, Bao NR, Wang S, Xi M, Zhang TH, Chen FS (2021) Ketamine induces ferroptosis of liver cancer cells by targeting lncRNA PVT1/miR-214–3p/GPX4. Drug Des Dev Ther 15:3965–3978. https://doi.org/10.2147/DDDT.S332847
Li X, Li Y, Lian P, Lv Q, Liu F (2023) Silencing lncRNA HCG18 regulates GPX4-inhibited ferroptosis by adsorbing miR-450b-5p to avert sorafenib resistance in hepatocellular carcinoma. Hum Exp Toxicol 42:9603271221142818. https://doi.org/10.1177/09603271221142818
Guan L, Wang F, Wang M, Han S, Cui Z, Xi S et al (2022) Downregulation of HULC induces ferroptosis in hepatocellular carcinoma via targeting of the miR-3200–5p/ATF4 axis. Oxid Med Cell Longev 2022:9613095. https://doi.org/10.1155/2022/9613095
Shi Z, Li Z, Jin B, Ye W, Wang L, Zhang S et al (2023) Loss of LncRNA DUXAP8 synergistically enhanced sorafenib induced ferroptosis in hepatocellular carcinoma via SLC7A11 de-palmitoylation. Clin Transl Med 13:e1300. https://doi.org/10.1002/ctm2.1300
Zhang Y, Luo M, Cui X, O’Connell D, Yang Y (2022) Long noncoding RNA NEAT1 promotes ferroptosis by modulating the miR-362–3p/MIOX axis as a ceRNA. Cell Death Differ 29:1850–1863. https://doi.org/10.1038/s41418-022-00970-9
Zhang B, Bao W, Zhang S, Chen B, Zhou X, Zhao J et al (2022) LncRNA HEPFAL accelerates ferroptosis in hepatocellular carcinoma by regulating SLC7A11 ubiquitination. Cell Death Dis 13:734. https://doi.org/10.1038/s41419-022-05173-1
Kang X, Huo Y, Jia S, He F, Li H, Zhou Q et al (2022) Silenced LINC01134 enhances oxaliplatin sensitivity by facilitating ferroptosis through GPX4 in hepatocarcinoma. Front Oncol 12:939605. https://doi.org/10.3389/fonc.2022.939605
Ren X, Wang X, Yan Y, Chen X, Cai Y, Liang Q et al (2022) Integrative bioinformatics and experimental analysis revealed TEAD as novel prognostic target for hepatocellular carcinoma and its roles in ferroptosis regulation. Aging 14:961–974. https://doi.org/10.18632/aging.203853
Qin Y, Pei Z, Feng Z, Lin P, Wang S, Li Y et al (2021) Oncogenic activation of YAP signaling sensitizes ferroptosis of hepatocellular carcinoma via ALOXE3-mediated lipid peroxidation accumulation. Front Cell Dev Biol 9:751593. https://doi.org/10.3389/fcell.2021.751593
Ascenzi F, De Vitis C, Maugeri-Saccà M, Napoli C, Ciliberto G, Mancini R (2021) SCD1, autophagy and cancer: implications for therapy. J Exp Clin Cancer Res 40:265. https://doi.org/10.1186/s13046-021-02067-6
Luis G, Godfroid A, Nishiumi S, Cimino J, Blacher S, Maquoi E et al (2021) Tumor resistance to ferroptosis driven by Stearoyl-CoA Desaturase-1 (SCD1) in cancer cells and Fatty Acid Biding Protein-4 (FABP4) in tumor microenvironment promote tumor recurrence. Redox Biol 43:102006. https://doi.org/10.1016/j.redox.2021.102006
Zhao Y, Li M, Yao X, Fei Y, Lin Z, Li Z et al (2020) HCAR1/MCT1 regulates tumor ferroptosis through the lactate-mediated AMPK-SCD1 activity and its therapeutic implications. Cell Rep 33:108487. https://doi.org/10.1016/j.celrep.2020.108487
Zhang L, Li XM, Shi XH, Ye K, Fu XL, Wang X et al (2022) Sorafenib triggers ferroptosis via inhibition of HBXIP/SCD axis in hepatocellular carcinoma. Acta Pharmacol Sin. https://doi.org/10.1038/s41401-022-00981-9
Xu R, Wang W, Zhang W (2023) Ferroptosis and the bidirectional regulatory factor p53. Cell Death Discov 9:197. https://doi.org/10.1038/s41420-023-01517-8
Zhang Z, Guo M, Shen M, Kong D, Zhang F, Shao J et al (2020) The BRD7-P53-SLC25A28 axis regulates ferroptosis in hepatic stellate cells. Redox Biol 36:101619. https://doi.org/10.1016/j.redox.2020.101619
Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H et al (2015) Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520:57–62. https://doi.org/10.1038/nature14344
Ou Y, Wang SJ, Li D, Chu B, Gu W (2016) Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc Natl Acad Sci USA 113:E6806–E6812. https://doi.org/10.1073/pnas.1607152113
Xie Y, Zhu S, Song X, Sun X, Fan Y, Liu J et al (2017) The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity. Cell Rep 20:1692–1704. https://doi.org/10.1016/j.celrep.2017.07.055
Zhang X, Zheng Q, Yue X, Yuan Z, Ling J, Yuan Y et al (2022) ZNF498 promotes hepatocellular carcinogenesis by suppressing p53-mediated apoptosis and ferroptosis via the attenuation of p53 Ser46 phosphorylation. J Exp Clin Cancer Res 41:79. https://doi.org/10.1186/s13046-022-02288-3
Chen J, Zhao F, Yang H, Wen J, Tang Y, Wan F et al (2022) Gentian violet induces apoptosis and ferroptosis via modulating p53 and MDM2 in hepatocellular carcinoma. Am J Cancer Res 12:3357–3372
Ghosh R, Samanta P, Sarkar R, Biswas S, Saha P, Hajra S et al (2022) Targeting HIF-1α by natural and synthetic compounds: a promising approach for anti-cancer therapeutics development. Molecules 27:5192. https://doi.org/10.3390/molecules27165192
Liu Y, He L, Liu B, Ying Y, Xu J, Yu M et al (2022) Pharmacological inhibition of sphingolipid synthesis reduces ferroptosis by stimulating the HIF-1 pathway. iScience 25:104533. https://doi.org/10.1016/j.isci.2022.104533
Yang M, Wu X, Hu J, Wang Y, Wang Y, Zhang L et al (2022) COMMD10 inhibits HIF1α/CP loop to enhance ferroptosis and radiosensitivity by disrupting Cu-Fe balance in hepatocellular carcinoma. J Hepatol 76:1138–1150. https://doi.org/10.1016/j.jhep.2022.01.009
Li Y, Yang W, Zheng Y, Dai W, Ji J, Wu L et al (2023) Targeting fatty acid synthase modulates sensitivity of hepatocellular carcinoma to sorafenib via ferroptosis. J Exp Clin Cancer Res 42:6. https://doi.org/10.1186/s13046-022-02567-z
Fan Z, Yang G, Zhang W, Liu Q, Liu G, Liu P et al (2021) Hypoxia blocks ferroptosis of hepatocellular carcinoma via suppression of METTL14 triggered YTHDF2-dependent silencing of SLC7A11. J Cell Mol Med 25:10197–10212. https://doi.org/10.1111/jcmm.16957
Wu J, Xue R, Wu M, Yin X, Xie B, Meng Q (2022) Nrf2-mediated ferroptosis inhibition exerts a protective effect on acute-on-chronic liver failure. Oxid Med Cell Longev 2022:4505513. https://doi.org/10.1155/2022/4505513
Shan Y, Yang G, Lu Q, Hu X, Qi D, Zhou Y et al (2022) Centrosomal protein 290 is a novel prognostic indicator that modulates liver cancer cell ferroptosis via the Nrf2 pathway. Aging 14:2367–2382. https://doi.org/10.18632/aging.203946
Tang K, Chen Q, Liu Y, Wang L, Lu W (2022) Combination of metformin and sorafenib induces ferroptosis of hepatocellular carcinoma through p62-Keap1-Nrf2 pathway. J Cancer 13:3234–3243. https://doi.org/10.7150/jca.76618
Elkateb AS, Nofal S, Ali SA, Atya HB (2023) Camptothecin sensitizes hepatocellular carcinoma cells to sorafenib- induced ferroptosis via suppression of Nrf2. Inflammation 46:1493–1511. https://doi.org/10.1007/s10753-023-01823-4
Wang Q, Bin C, Xue Q, Gao Q, Huang A, Wang K et al (2021) GSTZ1 sensitizes hepatocellular carcinoma cells to sorafenib-induced ferroptosis via inhibition of NRF2/GPX4 axis. Cell Death Dis 12:426. https://doi.org/10.1038/s41419-021-03718-4
Feng H, Liu Y, Gan Y, Li M, Liu R, Liang Z et al (2022) AdipoR1 regulates ionizing radiation-induced ferroptosis in HCC cells through Nrf2/xCT pathway. Oxid Med Cell Longev 2022:8091464. https://doi.org/10.1155/2022/8091464
Zhao Y, Wang Y, Miao Z, Liu Y, Yang Q (2023) c-Myc protects hepatocellular carcinoma cell from ferroptosis induced by glutamine deprivation via upregulating GOT1 and Nrf2. Mol Biol Rep 50:6627–6641. https://doi.org/10.1007/s11033-023-08495-1
Li H, Zhao J, Zhong XL, Xu PY, Du LJ, Fang P et al (2023) CPLX2 regulates ferroptosis and apoptosis through NRF2 pathway in human hepatocellular carcinoma cells. Appl Biochem Biotechnol 195:597–609. https://doi.org/10.1007/s12010-022-04135-9
Zhu YJ, Zheng B, Wang HY, Chen L (2017) New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta Pharmacol Sin 38:614–622. https://doi.org/10.1038/aps.2017.5
Huang W, Chen K, Lu Y, Zhang D, Cheng Y, Li L et al (2021) ABCC5 facilitates the acquired resistance of sorafenib through the inhibition of SLC7A11-induced ferroptosis in hepatocellular carcinoma. Neoplasia 23:1227–1239. https://doi.org/10.1016/j.neo.2021.11.002
Hsu TW, Su YH, Chen HA, Liao PH, Shen SC, Tsai KY et al (2023) Galectin-1-mediated MET/AXL signaling enhances sorafenib resistance in hepatocellular carcinoma by escaping ferroptosis. Aging 15:6503–6525. https://doi.org/10.18632/aging.204867
Huang CY, Chen LJ, Chen G, Chao TI, Wang CY (2022) SHP-1/STAT3-signaling-axis-regulated coupling between BECN1 and SLC7A11 contributes to sorafenib-induced ferroptosis in hepatocellular carcinoma. Int J Mol Sci. https://doi.org/10.3390/ijms231911092
Hua HW, Jiang HS, Jia L, Jia YP, Yao YL, Chen YW et al (2021) SPARC regulates ferroptosis induced by sorafenib in human hepatocellular carcinoma. Cancer Biomark 32:425–433. https://doi.org/10.3233/CBM-200101
Louandre C, Marcq I, Bouhlal H, Lachaier E, Godin C, Saidak Z et al (2015) The retinoblastoma (Rb) protein regulates ferroptosis induced by sorafenib in human hepatocellular carcinoma cells. Cancer Lett 356:971–977. https://doi.org/10.1016/j.canlet.2014.11.014
Li B, Wei S, Yang L, Peng X, Ma Y, Wu B et al (2021) CISD2 promotes resistance to sorafenib-induced ferroptosis by regulating autophagy in hepatocellular carcinoma. Front Oncol 11:657723. https://doi.org/10.3389/fonc.2021.657723
Wang K, Zhang Z, Tsai HI, Liu Y, Gao J, Wang M et al (2021) Branched-chain amino acid aminotransferase 2 regulates ferroptotic cell death in cancer cells. Cell Death Differ 28:1222–1236. https://doi.org/10.1038/s41418-020-00644-4
Zhao Y, Li Y, Zhang R, Wang F, Wang T, Jiao Y (2020) The role of erastin in ferroptosis and its prospects in cancer therapy. Onco Targets Ther 13:5429–5441. https://doi.org/10.2147/ott.S254995
Liao Y, Hu K, Liu W, Wang W, Qiu H, Pan S et al (2023) Bortezomib inhibits hepatocellular carcinoma via the Hippo-Yes-associated protein signalling pathway. Basic Clin Pharmacol Toxicol 132:297–311. https://doi.org/10.1111/bcpt.13832
Hu Z, Zhao Y, Li L, Jiang J, Li W, Mang Y et al (2023) Metformin promotes ferroptosis and sensitivity to sorafenib in hepatocellular carcinoma cells via ATF4/STAT3. Mol Biol Rep 50:6399–6413. https://doi.org/10.1007/s11033-023-08492-4
Zhang Y, Zhang H, Mu J, Han M, Cao Z, Dong F et al (2022) Eupalinolide B inhibits hepatic carcinoma by inducing ferroptosis and ROS-ER-JNK pathway. Acta Biochim Biophys Sin 54:974–986. https://doi.org/10.3724/abbs.2022082
Lin PL, Tang HH, Wu SY, Shaw NS, Su CL (2020) Saponin formosanin C-induced ferritinophagy and ferroptosis in human hepatocellular carcinoma cells. Antioxidants. https://doi.org/10.3390/antiox9080682
Huang D, Dong X, Li J, Chen Y, Zhou Y, Chen Q et al (2023) Steroidal saponin SSPH I induces ferroptosis in HepG2 cells via regulating iron metabolism. Med Oncol 40:132. https://doi.org/10.1007/s12032-023-02000-1
Chang WT, Bow YD, Fu PJ, Li CY, Wu CY, Chang YH et al (2021) A marine terpenoid, heteronemin, induces both the apoptosis and ferroptosis of hepatocellular carcinoma cells and involves the ROS and MAPK pathways. Oxid Med Cell Longev 2021:7689045. https://doi.org/10.1155/2021/7689045
Yao L, Yan D, Jiang B, Xue Q, Chen X, Huang Q et al (2023) Plumbagin is a novel GPX4 protein degrader that induces apoptosis in hepatocellular carcinoma cells. Free Radic Biol Med 203:1–10. https://doi.org/10.1016/j.freeradbiomed.2023.03.263
Xiu Z, Zhu Y, Han J, Li Y, Yang X, Yang G et al (2022) Caryophyllene oxide induces ferritinophagy by regulating the NCOA4/FTH1/LC3 pathway in hepatocellular carcinoma. Front Pharmacol 13:930958. https://doi.org/10.3389/fphar.2022.930958
Li ZJ, Dai HQ, Huang XW, Feng J, Deng JH, Wang ZX et al (2021) Artesunate synergizes with sorafenib to induce ferroptosis in hepatocellular carcinoma. Acta Pharmacol Sin 42:301–310. https://doi.org/10.1038/s41401-020-0478-3
Jin M, Shi C, Li T, Wu Y, Hu C, Huang G (2020) Solasonine promotes ferroptosis of hepatoma carcinoma cells via glutathione peroxidase 4-induced destruction of the glutathione redox system. Biomed Pharmacother 129:110282. https://doi.org/10.1016/j.biopha.2020.110282
Conche C, Finkelmeier F, Pešić M, Nicolas AM, Böttger TW, Kennel KB et al (2023) Combining ferroptosis induction with MDSC blockade renders primary tumours and metastases in liver sensitive to immune checkpoint blockade. Gut. https://doi.org/10.1136/gutjnl-2022-327909
Cheu JW, Lee D, Li Q, Goh CC, Bao MH, Yuen VW et al (2023) Ferroptosis suppressor protein 1 inhibition promotes tumor ferroptosis and anti-tumor immune responses in liver cancer. Cell Mol Gastroenterol Hepatol 16:133–159. https://doi.org/10.1016/j.jcmgh.2023.03.001
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–264. https://doi.org/10.1038/nrc3239
Ringelhan M, Pfister D, O’Connor T, Pikarsky E, Heikenwalder M (2018) The immunology of hepatocellular carcinoma. Nat Immunol 19:222–232. https://doi.org/10.1038/s41590-018-0044-z
Xu J, Lin X, Han T, Zhou Q, Su Y, Jiang S et al (2022) Regulation mechanism of ferroptosis and its research progress in tumor immunotherapy. Front Mol Biosci 9:1045548. https://doi.org/10.3389/fmolb.2022.1045548
Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK et al (2019) CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 569:270–274. https://doi.org/10.1038/s41586-019-1170-y
Hao X, Zheng Z, Liu H, Zhang Y, Kang J, Kong X et al (2022) Inhibition of APOC1 promotes the transformation of M2 into M1 macrophages via the ferroptosis pathway and enhances anti-PD1 immunotherapy in hepatocellular carcinoma based on single-cell RNA sequencing. Redox Biol 56:102463. https://doi.org/10.1016/j.redox.2022.102463
Ferrara M, Chialli G, Ferreira LM, Ruggieri E, Careccia G, Preti A et al (2020) Oxidation of HMGB1 is a dynamically regulated process in physiological and pathological conditions. Front Immunol 11:1122. https://doi.org/10.3389/fimmu.2020.01122
Chen Q, Zheng W, Guan J, Liu H, Dan Y, Zhu L et al (2023) SOCS2-enhanced ubiquitination of SLC7A11 promotes ferroptosis and radiosensitization in hepatocellular carcinoma. Cell Death Differ 30:137–151. https://doi.org/10.1038/s41418-022-01051-7
Zheng X, Liu B, Liu X, Li P, Zhang P, Ye F et al (2022) PERK regulates the sensitivity of hepatocellular carcinoma cells to high-LET carbon ions via either apoptosis or ferroptosis. J Cancer 13:669–680. https://doi.org/10.7150/jca.61622
Yuan Y, Cao W, Zhou H, Qian H, Wang H (2021) CLTRN, regulated by NRF1/RAN/DLD protein complex, enhances radiation sensitivity of hepatocellular carcinoma cells through ferroptosis pathway. Int J Radiat Oncol Biol Phys 110:859–871. https://doi.org/10.1016/j.ijrobp.2020.12.062
Xu M, Yang L, Lin Y, Lu Y, Bi X, Jiang T et al (2022) Emerging nanobiotechnology for precise theranostics of hepatocellular carcinoma. J Nanobiotechnol 20:427. https://doi.org/10.1186/s12951-022-01615-2
Zhao S, Zheng W, Yu C, Xu G, Zhang X, Pan C et al (2022) The role of ferroptosis in the treatment and drug resistance of hepatocellular carcinoma. Front Cell Dev Biol 10:845232. https://doi.org/10.3389/fcell.2022.845232
Xiao Y, Xu Z, Cheng Y, Huang R, Xie Y, Tsai HI et al (2023) Fe(3+)-binding transferrin nanovesicles encapsulating sorafenib induce ferroptosis in hepatocellular carcinoma. Biomater Res 27:63. https://doi.org/10.1186/s40824-023-00401-x
Tang H, Chen D, Li C, Zheng C, Wu X, Zhang Y et al (2019) Dual GSH-exhausting sorafenib loaded manganese-silica nanodrugs for inducing the ferroptosis of hepatocellular carcinoma cells. Int J Pharm 572:118782. https://doi.org/10.1016/j.ijpharm.2019.118782
Liu X, Zhu X, Qi X, Meng X, Xu K (2021) Co-administration of iRGD with sorafenib-loaded iron-based metal-organic framework as a targeted ferroptosis agent for liver cancer therapy. Int J Nanomed 16:1037–1050. https://doi.org/10.2147/ijn.S292528
Ou W, Mulik RS, Anwar A, McDonald JG, He X, Corbin IR (2017) Low-density lipoprotein docosahexaenoic acid nanoparticles induce ferroptotic cell death in hepatocellular carcinoma. Free Radic Biol Med 112:597–607. https://doi.org/10.1016/j.freeradbiomed.2017.09.002
Hassannia B, Wiernicki B, Ingold I, Qu F, Van Herck S, Tyurina YY et al (2018) Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J Clin Invest 128:3341–3355. https://doi.org/10.1172/JCI99032
Zhang Y, Tan H, Daniels JD, Zandkarimi F, Liu H, Brown LM et al (2019) Imidazole ketone erastin induces ferroptosis and slows tumor growth in a mouse lymphoma model. Cell Chem Biol 26:623–633. https://doi.org/10.1016/j.chembiol.2019.01.008
Yao X, Yang P, Jin Z, Jiang Q, Guo R, Xie R et al (2019) Multifunctional nanoplatform for photoacoustic imaging-guided combined therapy enhanced by CO induced ferroptosis. Biomaterials 197:268–283. https://doi.org/10.1016/j.biomaterials.2019.01.026
Funding
This work was supported by Key Medical Discipline of Hangzhou City (2021–2021); Key Medical Discipline of Zhejiang Province (2018-2-3); Key Laboratory of Clinical Cancer Pharmacology and Toxicology Research of Zhejiang Province (2020E10021); Zhejiang Province Medical and Health Science and Technology Program (2023KY933); Zhejiang Traditional Chinese Medicine Science and Technology Project (2023ZL565).
Author information
Authors and Affiliations
Contributions
QX and LR drafted the manuscript. NR, JP, YY and YZ finalized the paper and provided suggestions to improve it. GW revised the paper and supervised the paper. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Q., Ren, L., Ren, N. et al. Ferroptosis: a new promising target for hepatocellular carcinoma therapy. Mol Cell Biochem (2023). https://doi.org/10.1007/s11010-023-04893-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-023-04893-y