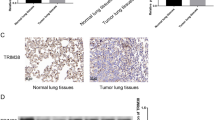
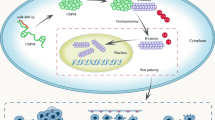
Abstract
The onset of non-small cell lung carcinoma (NSCLC) still be in the fog. LUCAT1 is potentially capable of modulating MCL-1-involved NSCLC pathogenesis via targeting SRSF1. Also, MCL-1 can regulate Wnt/β-catenin pathway to affect the tumorigenesis of NSCLC. Thus, this paper aims to uncover an intriguing and novel role of LUCAT1/SRSF1/MCL-1 axis in NSCLC based on Wnt/β-catenin pathway. A549 and NCI-H1650, two cell lines of NSCLC, were used to mimic NSCLC in vitro. MCL-1 siRNA (si-MCL-1) and LUCAT1 siRNA (si-LUCAT1) were used to downregulate MCL-1 and LUCAT1 in NSCLC cells, respectively. The overexpression vector of SRSF1 based on pcDNA 3.1 was constructed to upregulate SRSF1 expression. 40 μM SKL2001 was used to activate Wnt/β-catenin pathway. Transwell assay was used for migrative and invasive tests. The effect of LUCAT1 on tumor metastasis was verified in nude mice. MCL-1 downregulation led to the decrease of EMT, invasion, and migration in NSCLC, while Wnt/β-catenin pathway agonist partially reversed the effects of MCL-1 downregulation. Mechanistic investigations revealed that LUCAT1 and MCL-1 mRNA were enriched in SRSF1; LUCAT1 silence decreased MCL-1, whereas SRSF1 enhancement elevated MCL-1; Importantly, SRSF1 overexpression significantly reversed MCL-1 alteration due to LUCAT1 silence. In NSCLC cells, SRSF1 overexpression offset the si-LUCAT1-induced changes, and si-MCL-1 reversed the SRSF1-induced cellular changes. Further, LUCAT1 inhibition reduced lung metastasis of cancer cells. LUCAT1 can interact with SRSF1 to regulate MCL-1 expression that targets the Wnt/β-catenin pathway-mediated NSCLC cell migration, invasion, and EMT.







Similar content being viewed by others
Data Availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Chen P, Liu Y, Wen Y, Zhou C (2022) Non-small cell lung cancer in China. Cancer Commun (Lond) 42:937–970
Adderley H, Blackhall FH, Lindsay CR (2019) KRAS-mutant non-small cell lung cancer: converging small molecules and immune checkpoint inhibition. EBioMedicine 41:711–716
He J, Huang Z, Han L, Gong Y, Xie C (2021) Mechanisms and management of 3rd-generation EGFR-TKI resistance in advanced non-small cell lung cancer (Review). Int J Oncol 59:90
Sekihara K, Hishida T, Yoshida J, Oki T, Omori T, Katsumata S, Ueda T, Miyoshi T, Goto M, Nakasone S, Ichikawa T, Matsuzawa R, Aokage K, Goto K, Tsuboi M (2017) Long-term survival outcome after postoperative recurrence of non-small-cell lung cancer: who is “cured” from postoperative recurrence? Eur J Cardiothorac Surg 52:522–528
Deng HY, Zheng X, Zha P, Peng L, Huang KL, Qiu XM (2019) Diabetes mellitus and survival of non-small cell lung cancer patients after surgery: a comprehensive systematic review and meta-analysis. Thorac Cancer 10:571–578
Wang H, Guo M, Wei H, Chen Y (2021) Targeting MCL-1 in cancer: current status and perspectives. J Hematol Oncol 14:67
Tang Y, Yang Y, Luo J, Liu S, Zhan Y, Zang H, Zheng H, Zhang Y, Feng J, Fan S, Wen Q (2021) Overexpression of HSP10 correlates with HSP60 and Mcl-1 levels and predicts poor prognosis in non-small cell lung cancer patients. Cancer Biomark 30:85–94
Nakano T, Go T, Nakashima N, Liu D, Yokomise H (2020) Overexpression of antiapoptotic MCL-1 predicts worse overall survival of patients with non-small cell lung cancer. Anticancer Res 40:1007–1014
Shen Q, Xu Z, Xu S (2020) Long non-coding RNA LUCAT1 contributes to cisplatin resistance by regulating the miR-514a-3p/ULK1 axis in human non-small cell lung cancer. Int J Oncol 57:967–979
Sun Y, Jin SD, Zhu Q, Han L, Feng J, Lu XY, Wang W, Wang F, Guo RH (2017) Long non-coding RNA LUCAT1 is associated with poor prognosis in human non-small lung cancer and regulates cell proliferation via epigenetically repressing p21 and p57 expression. Oncotarget 8:28297–28311
Zhou X, Wang R, Li X, Yu L, Hua D, Sun C, Shi C, Luo W, Rao C, Jiang Z, Feng Y, Wang Q, Yu S (2019) Splicing factor SRSF1 promotes gliomagenesis via oncogenic splice-switching of MYO1B. J Clin Invest 129:676–693
Du JX, Luo YH, Zhang SJ, Wang B, Chen C, Zhu GQ, Zhu P, Cai CZ, Wan JL, Cai JL, Chen SP, Dai Z, Zhu W (2021) Splicing factor SRSF1 promotes breast cancer progression via oncogenic splice switching of PTPMT1. J Exp Clin Cancer Res 40:171
Liu J, Zhang Q, Ma N (2020) LncRNA GASAL1 interacts with SRSF1 to regulate trophoblast cell proliferation, invasion, and apoptosis via the mTOR signaling pathway. Cell Transplant 29:963689720965182
Yu F, Yu C, Li F, Zuo Y, Wang Y, Yao L, Wu C, Wang C, Ye L (2021) Wnt/beta-catenin signaling in cancers and targeted therapies. Signal Transduct Target Ther 6:307
Or CR, Huang CW, Chang CC, Lai YC, Chen YJ, Chang CC (2020) Obatoclax, a Pan-BCL-2 inhibitor, downregulates survivin to induce apoptosis in human colorectal carcinoma cells via suppressing WNT/beta-catenin signaling. Int J Mol Sci 21:1773
Tang Y, Yang P, Zhu Y, Su Y (2020) LncRNA TUG1 contributes to ESCC progression via regulating miR-148a-3p/MCL-1/Wnt/beta-catenin axis in vitro. Thorac Cancer 11:82–94
Carpenter R, Brady MF (2022) BAX gene. StatPearls, Treasure Island
Ferro F, Servais S, Besson P, Roger S, Dumas JF, Brisson L (2020) Autophagy and mitophagy in cancer metabolic remodelling. Semin Cell Dev Biol 98:129–138
Cen X, Chen Y, Xu X, Wu R, He F, Zhao Q, Sun Q, Yi C, Wu J, Najafov A, Xia H (2020) Pharmacological targeting of MCL-1 promotes mitophagy and improves disease pathologies in an Alzheimer’s disease mouse model. Nat Commun 11:5731
Yang L, Perez AA, Fujie S, Warden C, Li J, Wang Y, Yung B, Chen YR, Liu X, Zhang H, Zheng S, Liu Z, Ann D, Yen Y (2014) Wnt modulates MCL1 to control cell survival in triple negative breast cancer. BMC Cancer 14:124
Qi Z, Wang F, Yu G, Wang D, Yao Y, You M, Liu J, Liu J, Sun Z, Ji C, Xue Y, Yu S (2021) SRSF1 serves as a critical posttranscriptional regulator at the late stage of thymocyte development. Sci Adv 7:eabf0753
Ning X, Zhao J, He F, Yuan Y, Li B, Ruan J (2022) lncRNA NUTM2A-AS1 targets the SRSF1/Trim37 signaling pathway to promote the proliferation and invasion of breast cancer. Comput Math Methods Med 2022:3299336
Liu J, Li Y, Tong J, Gao J, Guo Q, Zhang L, Wang B, Zhao H, Wang H, Jiang E, Kurita R, Nakamura Y, Tanabe O, Engel JD, Bresnick EH, Zhou J, Shi L (2018) Long non-coding RNA-dependent mechanism to regulate heme biosynthesis and erythrocyte development. Nat Commun 9:4386
Ginn L, Shi L, Montagna M, Garofalo M (2020) LncRNAs in non-small-cell lung cancer. Noncoding RNA 6:25
Ghafouri-Fard S, Shoorei H, Branicki W, Taheri M (2020) Non-coding RNA profile in lung cancer. Exp Mol Pathol 114:104411
Xing C, Sun SG, Yue ZQ, Bai F (2021) Role of lncRNA LUCAT1 in cancer. Biomed Pharmacother 134:111158
Acknowledgements
We would like to thank the anonymous reviewers who have helped to improve the paper.
Funding
This work was supported by Guangxi Qihuang Scholar Cultivation Project, the Youth Science Funding Project of National Natural Science Foundation of China (82004296), the Key Subject Construction Project of Guangxi Traditional Chinese Medicine (GZXK-P-20-07), the Doctoral Scientific Research Foundation of Guangxi University of Chinese Medicine (2020BS029), the Youth Foundation of Self-funded Scientific Research Subject of Guangxi Health Commission (Z20200260), the Doctoral Scientific Research Foundation of The First Affiliated Hospital of Guangxi University of Chinese Medicine (2020BS006), and the Self-funded Scientific Research Subject of Guangxi Administration of Traditional Chinese Medicine (GXZYA20220073).
Author information
Authors and Affiliations
Contributions
Guarantor of integrity of the entire study: Sheng Xie. Study concepts: Wei Shi, Jiaxiao Wang. Study design: Fang Fang. Definition of intellectual content: Fang Fang. Literature research: Xiaowei Jin. Experimental studies: Fang Fang, Xiaowei Jin, Zhixin Dong, Jinming Meng. Data acquisition: Mei Zhao, Jinming Meng. Data analysis: Mei Zhao. Statistical analysis: Mei Zhao. Manuscript preparation: Fang Fang. Manuscript editing: Fang Fang. Manuscript review: Fang Fang
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Approval
The animal experimental protocol was approved by the Ethics Committee of The First Affiliated Hospital of Guangxi University of Chinese Medicine.
Consent to Participate
Not Applicable. This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to Publish
Not Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fang, F., Zhao, M., Jin, X. et al. Upregulation of MCL-1 by LUCAT1 through interacting with SRSF1 promotes the migration and invasion in non-small cell lung carcinoma. Mol Cell Biochem (2023). https://doi.org/10.1007/s11010-023-04851-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-023-04851-8