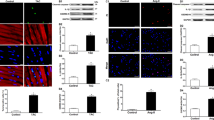
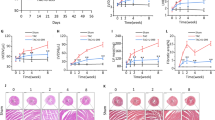
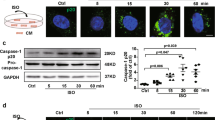
Abstract
Cardiac hypertrophy is the main adaptive response of the heart to chronic loads; however, prolonged or excessive hypertrophy promotes myocardial interstitial fibrosis, systolic dysfunction, and cardiomyocyte death, especially aseptic inflammation mediated by NLRP3 inflammasome, which can aggravate ventricular remodeling and myocardial damage, which is an important mechanism for the progression of heart failure. Various cardiac overloads can cause mitochondrial damage. In recent years, the mitochondria have been demonstrated to be involved in the inflammatory response during the development of cardiac hypertrophy in vitro and in vivo. As the NLRP3 inflammasome and mitochondria are regulators of inflammation and cardiac hypertrophy, we explored the potential functions of the NLRP3 inflammasome and mitochondrial dysfunction in cardiac hypertrophy. In particular, we proposed that the induction of mitochondrial dysfunction in cardiomyocytes may promote NLRP3-dependent inflammation during myocardial hypertrophy. Further in-depth studies could prompt valuable discoveries regarding the underlying molecular mechanisms of cardiac hypertrophy, reveal novel anti-inflammatory therapies for cardiac hypertrophy, and provide more desirable therapeutic outcomes for patients with cardiac hypertrophy.



Similar content being viewed by others
Data availability
Enquiries about data availability should be directed to the authors.
Abbreviations
- ACEI:
-
Angiotensin-converting enzyme
- ASC:
-
Apoptosis-associated speck-like protein containing a CARD
- ATP:
-
Adenosine triphosphate
- DAMPs:
-
Danger-associated molecular patterns
- GSDMD:
-
Gasdermin D
- GSDMD-NT:
-
GSDMD-N-terminal
- IF1:
-
Inhibitory factor 1
- IL-1β:
-
Interleukin-1β
- IL-1R1:
-
IL-1 receptor type 1
- IL-18:
-
Interleukin-18
- IRI:
-
Ischemia-reperfusion injury
- mtDNA:
-
Mitochondrial DNA
- mt-dynamics:
-
Mitochondrial dynamics
- NF-κB:
-
Nuclear factor-κB
- NLRP3:
-
The pyridine domain-containing 3
- NLRs:
-
Nucleotide-binding and oligomerization domain (NOD)-like receptor
- NOX:
-
Nicotinamide adenine dinucleotide phosphate oxidase
- Ox-mtDNA:
-
Oxidation of mtDNA
- PPQ:
-
Pyrroloquinoline quinone
- ROS:
-
Reactive oxygen species
- SiNP:
-
Silica nanoparticle
- SUMO:
-
Small ubiquitin-related modifier
- TNF:
-
Tumor necrosis factor
- TNFR:
-
Tumor necrosis factor receptor
- TLR4:
-
Toll-like receptor 4
- YLLs:
-
Years of life lost
References
Joseph P et al (2017) Reducing the global burden of cardiovascular disease, Part 1: the epidemiology and risk factors. Circ Res 121(6):677–694
Gregory AR et al (2017) Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study. Lancet 392(10159):1736–1788
Yu W, Chen C, Cheng J (2020) The role and molecular mechanism of FoxO1 in mediating cardiac hypertrophy. ESC Heart Fail 7(6):3497–3504
Mele M et al (2021) Diagnosis of acute myocardial infarction in the time of the COVID-19 pandemic. Eur Heart J 42(3):286
Shah AK et al (2021) Oxidative stress as a mechanism for functional alterations in cardiac hypertrophy and heart failure. Antioxidants (Basel) 10(6):931
Verma SK et al (2012) Interleukin-10 treatment attenuates pressure overload-induced hypertrophic remodeling and improves heart function via signal transducers and activators of transcription 3-dependent inhibition of nuclear factor-κB. Circulation 126(4):418–429
van Hout GP et al (2017) The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. Eur Heart J 38(11):828–836
van Hout GPJ, Bosch L (2018) The inflammasomes in cardiovascular disease. Exp Suppl 108:9–40
Dorn GW 2nd, Vega RB, Kelly DP (2015) Mitochondrial biogenesis and dynamics in the developing and diseased heart. Genes Dev 29(19):1981–1991
Oldfield CJ, Duhamel TA, Dhalla NS (2020) Mechanisms for the transition from physiological to pathological cardiac hypertrophy. Can J Physiol Pharmacol 98(2):74–84
Fioranelli M et al (2021) Regulation of inflammatory reaction in health and disease. Int J Mol Sci 22(10):5277
Shen H, Kreisel D, Goldstein DR (2013) Processes of sterile inflammation. J Immunol 191(6):2857–2863
Man SM, Kanneganti TD (2015) Regulation of inflammasome activation. Immunol Rev 265(1):6–21
Patel MN et al (2017) Inflammasome priming in sterile inflammatory disease. Trends Mol Med 23(2):165–180
Bauernfeind FG et al (2009) Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 183(2):787–791
Swanson KV, Deng M, Ting JP (2019) The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol 19(8):477–489
Muñoz-Planillo R et al (2013) K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38(6):1142–1153
Murakami T et al (2012) Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci U S A 109(28):11282–11287
Verhoef PA et al (2005) Inhibitory effects of chloride on the activation of caspase-1, IL-1beta secretion, and cytolysis by the P2X7 receptor. J Immunol 175(11):7623–7634
Liu Z et al (2018) Silymarin attenuated paraquat-induced cytotoxicity in macrophage by regulating Trx/TXNIP complex, inhibiting NLRP3 inflammasome activation and apoptosis. Toxicol In Vitro 46:265–272
Shimada K et al (2012) Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36(3):401–414
Iyer SS et al (2013) Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 39(2):311–323
Hornung V et al (2008) Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 9(8):847–856
Grebe A, Hoss F, Latz E (2018) NLRP3 inflammasome and the IL-1 pathway in atherosclerosis. Circ Res 122(12):1722–1740
Weber ANR et al (2020) Recent insights into the regulatory networks of NLRP3 inflammasome activation. J Cell Sci. https://doi.org/10.1242/jcs.248344
Elliott EI, Sutterwala FS (2015) Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev 265(1):35–52
Zhou LY et al (2013) Mitochondrial function in cardiac hypertrophy. Int J Cardiol 167(4):1118–1125
Bergmann O et al (2009) Evidence for cardiomyocyte renewal in humans. Science 324(5923):98–102
Heallen TR et al (2020) Determinants of cardiac growth and size. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a037150
Tang X et al (2020) SNO-MLP (S-Nitrosylation of Muscle LIM Protein) facilitates myocardial hypertrophy through TLR3 (Toll-Like Receptor 3)-mediated RIP3 (Receptor-Interacting Protein Kinase 3) and NLRP3 (NOD-Like Receptor Pyrin Domain Containing 3) inflammasome activation. Circulation 141(12):984–1000
Dong Y et al (2018) Non-coding RNA-linked epigenetic regulation in cardiac hypertrophy. Int J Biol Sci 14(9):1133–1141
Stafford N et al (2020) Signaling via the Interleukin-10 receptor attenuates cardiac hypertrophy in mice during pressure overload, but not isoproterenol infusion. Front Pharmacol 11:559220
Suetomi T et al (2018) Inflammation and NLRP3 inflammasome activation initiated in response to pressure overload by Ca(2+)/Calmodulin-dependent protein kinase II δ signaling in cardiomyocytes are essential for adverse cardiac remodeling. Circulation 138(22):2530–2544
Li F et al (2018) NLRP3 deficiency accelerates pressure overload-induced cardiac remodeling via increased TLR4 expression. J Mol Med (Berl) 96(11):1189–1202
Pan XC et al (2019) Dual role of triptolide in interrupting the NLRP3 inflammasome pathway to attenuate cardiac fibrosis. Int J Mol Sci 20(2):360
Zhang T et al (2020) Astragaloside IV prevents myocardial hypertrophy induced by mechanical stress by activating autophagy and reducing inflammation. Am J Transl Res 12(9):5332–5342
Kubota T et al (1997) Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ Res 81(4):627–635
Meléndez GC et al (2010) Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension 56(2):225–231
Palmer JN et al (1995) Interleukin-1 beta induces cardiac myocyte growth but inhibits cardiac fibroblast proliferation in culture. J Clin Invest 95(6):2555–2564
Sun M et al (2007) Tumor necrosis factor-alpha mediates cardiac remodeling and ventricular dysfunction after pressure overload state. Circulation 115(11):1398–1407
Ma F et al (2012) Macrophage-stimulated cardiac fibroblast production of IL-6 is essential for TGF β/Smad activation and cardiac fibrosis induced by angiotensin II. PLoS ONE 7(5):e35144
Honsho S et al (2009) Pressure-mediated hypertrophy and mechanical stretch induces IL-1 release and subsequent IGF-1 generation to maintain compensative hypertrophy by affecting Akt and JNK pathways. Circ Res 105(11):1149–1158
Yu Q et al (2009) IL-18 induction of osteopontin mediates cardiac fibrosis and diastolic dysfunction in mice. Am J Physiol Heart Circ Physiol 297(1):H76-85
Pinto AR et al (2016) Revisiting cardiac cellular composition. Circ Res 118(3):400–409
Hu Y et al (2021) The AMPK-MFN2 axis regulates MAM dynamics and autophagy induced by energy stresses. Autophagy 17(5):1142–1156
van der Bliek AM, Shen Q, Kawajiri S (2013) Mechanisms of mitochondrial fission and fusion. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a011072
Chistiakov DA et al (2018) The role of mitochondrial dysfunction in cardiovascular disease: a brief review. Ann Med 50(2):121–127
Stanley WC, Recchia FA, Lopaschuk GD (2005) Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85(3):1093–1129
Siasos G et al (2018) Mitochondria and cardiovascular diseases-from pathophysiology to treatment. Ann Transl Med 6(12):256
Yu SB, Pekkurnaz G (2018) Mechanisms orchestrating mitochondrial dynamics for energy homeostasis. J Mol Biol 430(21):3922–3941
Jin JY et al (2021) Drp1-dependent mitochondrial fission in cardiovascular disease. Acta Pharmacol Sin 42(5):655–664
Fang L et al (2007) Down-regulation of mitofusin-2 expression in cardiac hypertrophy in vitro and in vivo. Life Sci 80(23):2154–2160
Yu H et al (2011) Mitofusin 2 inhibits angiotensin II-induced myocardial hypertrophy. J Cardiovasc Pharmacol Ther 16(2):205–211
Piquereau J et al (2012) Down-regulation of OPA1 alters mouse mitochondrial morphology, PTP function, and cardiac adaptation to pressure overload. Cardiovasc Res 94(3):408–417
Wai T et al (2015) Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science 350(6265):aad0116
Hasan P et al (2018) Mitochondrial fission protein, dynamin-related protein 1, contributes to the promotion of hypertensive cardiac hypertrophy and fibrosis in Dahl-salt sensitive rats. J Mol Cell Cardiol 121:103–106
Sandoval H et al (2008) Essential role for Nix in autophagic maturation of erythroid cells. Nature 454(7201):232–235
Yang K et al (2017) Knockout of the ATPase inhibitory factor 1 protects the heart from pressure overload-induced cardiac hypertrophy. Sci Rep 7(1):10501
Choubey V, Zeb A, Kaasik A (2021) Molecular mechanisms and regulation of mammalian mitophagy. Cells 11(1):38
Abudureyimu M et al (2020) Berberine promotes cardiac function by upregulating PINK1/Parkin-mediated mitophagy in heart failure. Front Physiol 11:565751
Song M et al (2014) Super-suppression of mitochondrial reactive oxygen species signaling impairs compensatory autophagy in primary mitophagic cardiomyopathy. Circ Res 115(3):348–353
Tong M et al (2019) Mitophagy is essential for maintaining cardiac function during high fat diet-induced diabetic cardiomyopathy. Circ Res 124(9):1360–1371
Seddon M, Looi YH, Shah AM (2007) Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart 93(8):903–907
Nakamura K et al (1998) Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-alpha and angiotensin II. Circulation 98(8):794–799
Pimentel DR et al (2001) Reactive oxygen species mediate amplitude-dependent hypertrophic and apoptotic responses to mechanical stretch in cardiac myocytes. Circ Res 89(5):453–460
Zhang GX et al (2005) Cardiac oxidative stress in acute and chronic isoproterenol-infused rats. Cardiovasc Res 65(1):230–238
Li HL et al (2006) Epigallocathechin-3 gallate inhibits cardiac hypertrophy through blocking reactive oxidative species-dependent and -independent signal pathways. Free Radic Biol Med 40(10):1756–1775
Guo J et al (2009) p66Shc links alpha1-adrenergic receptors to a reactive oxygen species-dependent AKT-FOXO3A phosphorylation pathway in cardiomyocytes. Circ Res 104(5):660–669
Okabe K et al (2020) DPP (Dipeptidyl Peptidase)-4 inhibitor attenuates Ang II (Angiotensin II)-induced cardiac hypertrophy via GLP (Glucagon-Like Peptide)-1-dependent suppression of Nox (Nicotinamide Adenine Dinucleotide Phosphate Oxidase) 4-HDAC (Histone Deacetylase) 4 pathway. Hypertension 75(4):991–1001
Novoa U et al (2017) High-intensity exercise reduces cardiac fibrosis and hypertrophy but does not restore the nitroso-redox imbalance in diabetic cardiomyopathy. Oxid Med Cell Longev 2017:7921363
Ma T et al (2019) TRPC3 deficiency attenuates high salt-induced cardiac hypertrophy by alleviating cardiac mitochondrial dysfunction. Biochem Biophys Res Commun 519(4):674–681
Xiong W et al (2018) PTEN induced putative kinase 1 (PINK1) alleviates angiotensin II-induced cardiac injury by ameliorating mitochondrial dysfunction. Int J Cardiol 266:198–205
Yu M et al (2012) Inhibitory effects of enalaprilat on rat cardiac fibroblast proliferation via ROS/P38MAPK/TGF-β1 signaling pathway. Molecules 17(3):2738–2751
Yan C et al (2019) Mitochondrial DNA: distribution, mutations, and elimination. Cells 8(4):379
Tsutsui H, Kinugawa S, Matsushima S (2009) Mitochondrial oxidative stress and dysfunction in myocardial remodelling. Cardiovasc Res 81(3):449–456
Dai DF, Rabinovitch P (2011) Mitochondrial oxidative stress mediates induction of autophagy and hypertrophy in angiotensin-II treated mouse hearts. Autophagy 7(8):917–918
Suárez-Rivero JM et al (2021) From mitochondria to atherosclerosis: the inflammation path. Biomedicines 9(3):258
Huang Y et al (2022) Brown adipose TRX2 deficiency activates mtDNA-NLRP3 to impair thermogenesis and protect against diet-induced insulin resistance. J Clin Invest. https://doi.org/10.1172/JCI148852
Nakahira K et al (2011) Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol 12(3):222–230
Liu Z et al (2022) XBP1 deficiency promotes hepatocyte pyroptosis by impairing mitophagy to activate mtDNA-cGAS-STING signaling in macrophages during acute liver injury. Redox Biol 52:102305
Ichinohe T et al (2013) Mitochondrial protein mitofusin 2 is required for NLRP3 inflammasome activation after RNA virus infection. Proc Natl Acad Sci U S A 110(44):17963–17968
Park S et al (2015) Defective mitochondrial fission augments NLRP3 inflammasome activation. Sci Rep 5:15489
Mahalanobish S et al (2020) Melatonin induced suppression of ER stress and mitochondrial dysfunction inhibited NLRP3 inflammasome activation in COPD mice. Food Chem Toxicol 144:111588
Zhang XL et al (2021) Anti-inflammatory and neuroprotective effects of natural cordycepin in rotenone-induced PD models through inhibiting Drp1-mediated mitochondrial fission. Neurotoxicology 84:1–13
Chen Y et al (2021) Nlrp3 deficiency alleviates angiotensin II-induced cardiomyopathy by inhibiting mitochondrial dysfunction. Oxid Med Cell Longev 2021:6679100
Alves JV et al (2020) Supraphysiological levels of testosterone induce vascular dysfunction via activation of the NLRP3 inflammasome. Front Immunol 11:1647
Papamitsou T et al (2011) Testosterone-induced hypertrophy, fibrosis and apoptosis of cardiac cells–an ultrastructural and immunohistochemical study. Med Sci Monit 17(9):Br266–Br273
Guo H et al (2017) NLRP3 deficiency attenuates renal fibrosis and ameliorates mitochondrial dysfunction in a mouse unilateral ureteral obstruction model of chronic kidney disease. Mediators Inflamm 2017:8316560
Ding W et al (2016) Mitochondrial reactive oxygen species-mediated NLRP3 inflammasome activation contributes to aldosterone-induced renal tubular cells injury. Oncotarget 7(14):17479–17491
Zhao M et al (2021) Selective inhibition of NLRP3 inflammasome reverses pressure overload-induced pathological cardiac remodeling by attenuating hypertrophy, fibrosis, and inflammation. Int Immunopharmacol 99:108046
Wang M et al (2022) MCC950, a selective NLRP3 inhibitor, attenuates adverse cardiac remodeling following heart failure through improving the cardiometabolic dysfunction in obese mice. Front Cardiovasc Med 9:727474
Shi Y et al (2022) The selective NLRP3 inflammasome inhibitor MCC950 improves isoproterenol-induced cardiac dysfunction by inhibiting cardiomyocyte senescence. Eur J Pharmacol 937:175364
Yue R et al (2021) NLRP3-mediated pyroptosis aggravates pressure overload-induced cardiac hypertrophy, fibrosis, and dysfunction in mice: cardioprotective role of irisin. Cell Death Discov 7(1):50
Li R et al (2017) Triptolide attenuates pressure overload-induced myocardial remodeling in mice via the inhibition of NLRP3 inflammasome expression. Biochem Biophys Res Commun 485(1):69–75
Ren B et al (2021) Ginsenoside Rg3 attenuates angiotensin II-induced myocardial hypertrophy through repressing NLRP3 inflammasome and oxidative stress via modulating SIRT1/NF-κB pathway. Int Immunopharmacol 98:107841
Lv SL et al (2021) Lp-PLA2 inhibition prevents Ang II-induced cardiac inflammation and fibrosis by blocking macrophage NLRP3 inflammasome activation. Acta Pharmacol Sin 42(12):2016–2032
Yan M et al (2022) The Chinese herbal medicine Fufang Zhenzhu Tiaozhi protects against diabetic cardiomyopathy by alleviating cardiac lipotoxicity-induced oxidative stress and NLRP3-dependent inflammasome activation. Biomed Pharmacother 148:112709
Wang F et al (2022) Silica nanoparticles induce pyroptosis and cardiac hypertrophy via ROS/NLRP3/Caspase-1 pathway. Free Radic Biol Med 182:171–181
Qu XF et al (2022) Pyrroloquinoline quinone ameliorates diabetic cardiomyopathy by inhibiting the pyroptosis signaling pathway in C57BL/6 mice and AC16 cells. Eur J Nutr 61(4):1823–1836
Funding
This work was supported by Jilin Provincial Science and Technology Commission International Science and Technology Cooperation (Grant No. 20230402015GH), Jilin Provincial Department of Finance (Grant No. 2021SCZ24), and Jilin Provincial Science and Technology Development Plan Project (Grant No. 20210101258JC).
Author information
Authors and Affiliations
Contributions
YM proposed conceptualization. RY drafted the manuscript. YS and YY assisted RY in screening articles. YJ and RZ created computer graphics and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, R., Sun, Y., Yang, Y. et al. Mitochondria and NLRP3 inflammasome in cardiac hypertrophy. Mol Cell Biochem (2023). https://doi.org/10.1007/s11010-023-04812-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-023-04812-1