Abstract
Iron is an essential trace element involved in oxidation–reduction reactions, oxygen transport and storage, and energy metabolism. Iron in excess can be toxic for cells, since iron produces reactive oxygen species and is important for survival of pathogenic microbes. There is a fine-tuning in the regulation of serum iron levels, determined by intestinal absorption, macrophage iron recycling, and mobilization of hepatocyte stores versus iron utilization, primarily by erythroid cells in the bone marrow. Hepcidin is the major regulatory hormone of systemic iron homeostasis and is upregulated during inflammation. Hepcidin metabolism is altered in chronic kidney disease. Ferroportin is an iron export protein and mediates iron release into the circulation from duodenal enterocytes, splenic reticuloendothelial macrophages, and hepatocytes. Systemic iron homeostasis is controlled by the hepcidin–ferroportin axis at the sites of iron entry into the circulation. Hepcidin binds to ferroportin, induces its internalization and intracellular degradation, and thus inhibits iron absorption from enterocytes, and iron release from macrophages and hepatocytes. Recent data suggest that hepcidin, by slowing or preventing the mobilization of iron from macrophages, may promote atherosclerosis and may be associated with increased cardiovascular disease risk. This article reviews the current data regarding the molecular and cellular pathways of systemic and autocrine hepcidin production and seeks the answer to the question whether changes in hepcidin translate into clinical outcomes of all-cause and cardiovascular mortality, and cardiovascular and renal end-points.



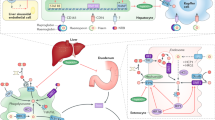
source of iron deposits within the plaques. CD36 is the major scavenger receptor for Ox-LDL. Expression of CD36 is upregulated by hepcidin and Ox-LDL. Cholesterol efflux in macrophages is by cholesterol transporters ATP-binding cassette subfamily A1 (ABCA1) and subfamily G1 (ABCG1). Ox-LDL uptake by macrophages results in induction of CD36 and peroxisome proliferator-activated receptor gamma (PPARγ), which then activates liver receptor X (LXR) to upregulate ABCA1 and ABCG1 gene expression, subsequently stimulating cellular cholesterol efflux mediated by apolipoprotein A-I (ApoA-I) and high-density lipoprotein (HDL), respectively. There is a vicious cycle between TLR-4/ NF-κB activation and autocrine formation of hepcidin-induced iron retention. Hepcidin suppresses Ox-LDL-induced upregulation of proteins involved in macrophage cholesterol efflux
Similar content being viewed by others
References
Eisenga MF, Dullaart RP, Berger SP, Sloan JH, De Vries AP, Bakker SJ, Gaillard CA (2016) Association of hepcidin-25 with survival after kidney transplantation. Eur J Clin Invest 46:994–1001. https://doi.org/10.1111/eci.12682
Vela D (2018) Balance of cardiac and systemic hepcidin and its role in heart physiology and pathology. Lab Invest 98:315–326. https://doi.org/10.1038/labinvest.2017.111
Babitt JL, Lin HY (2010) Molecular mechanisms of hepcidin regulation: implications for the anemia of CKD. Am J Kidney Dis 55:726–741. https://doi.org/10.1053/j.ajkd.2009.12.030
Lakhal-Littleton S, Wolna M, Chung YJ, Christian HC, Heather LC, Brescia M, Ball V, Diaz R, Santos A, Biggs D, Clarke K, Davies B, Robbins PA (2016) An essential cell-autonomous role for hepcidin in cardiac iron homeostasis. Elife 5:e19804. https://doi.org/10.7554/eLife.19804
Van der Weerd N, Grooteman M, Nube M, Ter Wee P, Swinkels D, Gaillard CA (2015) Hepcidin in chronic kidney disease: not an anaemia management tool, but promising as a cardiovascular biomarker. Neth J Med 73:108–118
van der Weerd NC, Grooteman MP, Bots ML, van den Dorpel MA, den Hoedt CH, Mazairac AH, Nubé MJ, Penne EL, Wetzels JF, Wiegerinck ET, Swinkels DW, Blankestijn PJ, Ter Wee PM, Investigators CONTRAST (2013) Hepcidin-25 is related to cardiovascular events in chronic haemodialysis patients. Nephrol Dial Transplant 28:3062–3071. https://doi.org/10.1093/ndt/gfs488
Li YQ, Bai B, Zheng QQ, Yan H, Zhuang GH (2013) Quantitative study of iron metabolism-related genes expression in rat. Biomed Environ Sci 26:808–819. https://doi.org/10.3967/bes2013.004
Merle U, Fein E, Gehrke SG, Stremmel W, Kulaksiz H (2007) The iron regulatory peptide hepcidin is expressed in the heart and regulated by hypoxia and inflammation. Endocrinology 148:2663–2668. https://doi.org/10.1210/en.2006-1331
Sheikh N, Dudas J, Ramadori G (2007) Changes of gene expression of iron regulatory proteins during turpentine oil-induced acute-phase response in the rat. Lab Invest 87:713–725. https://doi.org/10.1038/labinvest.3700553
Suzuki T, Hanawa H, Jiao S, Ohno Y, Hayashi Y, Yoshida K, Kashimura T, Obata H, Minamino T (2014) Inappropriate expression of hepcidin by liver congestion contributes to anemia and relative iron deficiency. J Card Fail 20:268–277. https://doi.org/10.1016/j.cardfail.2014.01.008
Ohno Y, Hanawa H, Jiao S, Hayashi Y, Yoshida K, Suzuki T, Kashimura T, Obata H, Tanaka K, Watanabe T, Minamino T (2015) Liver congestion in heart failure contributes to inappropriately increased serum hepcidin despite anemia. Tohoku J Exp Med 235:69–79. https://doi.org/10.1620/tjem.235.69
van Breda GF, Bongartz LG, Zhuang W, van Swelm RP, Pertijs J, Braam B, Cramer MJ, Swinkels DW, Doevendans PA, Verhaar MC, Masereeuw R, Joles JA, Gaillard CA (2016) Cardiac hepcidin expression associates with injury independent of iron. Am J Nephrol 44:368–378. https://doi.org/10.1159/000449419
Petrak J, Havlenova T, Krijt M, Behounek M, Franekova J, Cervenka L, Pluhacek T, Vyoral D, Melenovsky V (2019) Myocardial iron homeostasis and hepcidin expression in a rat model of heart failure at different levels of dietary iron intake. Biochim Biophys Acta Gen Subj 1863:703–713. https://doi.org/10.1016/j.bbagen.2019.01.010
Zlatanova I, Pinto C, Bonnin P, Mathieu JRR, Bakker W, Vilar J, Lemitre M, Voehringer D, Vaulont S, Peyssonnaux C, Silvestre JS (2019) Iron regulator hepcidin impairs macrophage-dependent cardiac repair after injury. Circulation 139:1530–1547. https://doi.org/10.1161/CIRCULATIONAHA.118.034545
Lakhal-Littleton S (2019) Cardiomyocyte hepcidin: From intracellular iron homeostasis to physiological function. Vitam Horm 110:189–200. https://doi.org/10.1016/bs.vh.2019.01.009
Daher R, Karim Z (2017) Iron metabolism: state of the art. Transfus Clin Biol 24:115–119. https://doi.org/10.1016/j.tracli.2017.06.015
Simonis G, Mueller K, Schwarz P, Wiedemann S, Adler G, Strasser RH, Kulaksiz H (2010) The iron-regulatory peptide hepcidin is upregulated in the ischemic and in the remote myocardium after myocardial infarction. Peptides 31:1786–1790. https://doi.org/10.1016/j.peptides.2010.05.013
Suzuki H, Toba K, Kato K, Ozawa T, Tomosugi N, Higuchi M, Kusuyama T, Iso Y, Kobayashi N, Yokoyama S, Fukuda N, Saitoh H, Akazawa K, Aizawa Y (2009) Serum hepcidin-20 is elevated during the acute phase of myocardial infarction. Tohoku J Exp Med 218:93–98. https://doi.org/10.1620/tjem.218.93
Isoda M, Hanawa H, Watanabe R, Yoshida T, Toba K, Yoshida K, Kojima M, Otaki K, Hao K, Ding L, Tanaka K, Takayama T, Kato K, Okura Y, Kodama M, Ota Y, Hayashi J, Aizawa Y (2010) Expression of the peptide hormone hepcidin increases in cardiomyocytes under myocarditis and myocardial infarction. J Nutr Biochem 21:749–756. https://doi.org/10.1016/j.jnutbio.2009.04.009
Naito Y, Hosokawa M, Sawada H, Oboshi M, Iwasaku T, Okuhara Y, Morisawa D, Eguchi A, Hirotani S, Ohyanagi M, Tsujino T, Masuyama T (2014) Hepcidin is increased in the hypertrophied heart of Dahl salt-sensitive rats. Int J Cardiol 172:e45–e47. https://doi.org/10.1016/j.ijcard.2013.12.067
Lakhal-Littleton S (2019) Mechanisms of cardiac iron homeostasis and their importance to heart function. Free Radic Biol Med 133:234–237. https://doi.org/10.1016/j.freeradbiomed.2018.08.010
Galesloot TE, Holewijn S, Kiemeney LA, de Graaf J, Vermeulen SH, Swinkels DW (2014) Serum hepcidin is associated with presence of plaque in postmenopausal women of a general population. Arterioscler Thromb Vasc Biol 34:446–456. https://doi.org/10.1161/ATVBAHA.113.302381
Pechlaner R, Kiechl S, Mayr M, Santer P, Weger S, Haschka D, Bansal SS, Willeit J, Weiss G (2016) Correlates of serum hepcidin levels and its association with cardiovascular disease in an elderly general population. Clin Chem Lab Med 54:151–161. https://doi.org/10.1515/cclm-2015-0068
Wang X, Sheng L, Ye P, Cao R, Yang X, Xiao W, Zhang Y, Bai Y, Wu H (2018) The association between Hepcidin and arterial stiffness in a community-dwelling population. Lipids Health Dis 17:244. https://doi.org/10.1186/s12944-018-0866-6
Xiao L, Luo G, Guo X, Jiang C, Zeng H, Zhou F, Li Y, Yu J, Yao P (2020) Macrophage iron retention aggravates atherosclerosis: evidence for the role of autocrine formation of hepcidin in plaque macrophages. Biochim Biophys Acta Mol Cell Biol Lipids 1865:158531. https://doi.org/10.1016/j.bbalip.2019.158531
Sullivan JL (2007) Macrophage iron, hepcidin, and atherosclerotic plaque stability. Exp Biol Med (Maywood) 232:1014–1020. https://doi.org/10.3181/0703-MR-54
Li X, Ding D, Zhang Y, Su D, Wang M, Chen X, Yang Y, Hong C, Hu G, Ling W (2017) Associations of plasma hepcidin with mortality risk in patients with coronary artery disease. Oncotarget 8(65):109497. https://doi.org/10.18632/oncotarget.22722
Haase-Fielitz A, Plaß M, Kuppe H, Hetzer R, Ostland V, Westphal S, Hoffmann J, Prowle J, Mertens PR, Westerman M, Bellomo R, Haase M (2013) Low preoperative hepcidin concentration as a risk factor for mortality after cardiac surgery: A pilot study. J Thorac Cardiovasc Surg 145:1380–1386. https://doi.org/10.1016/j.jtcvs.2012.09.003
Zeller T, Altay A, Waldeyer C, Appelbaum S, Ojeda F, Ruhe J, Schnabel RB, Lackner KJ, Blankenberg S, Karakas M (2018) Prognostic value of iron-homeostasis regulating peptide hepcidin in coronary heart disease—evidence from the large atherogene study. Biomolecules 8:43. https://doi.org/10.3390/biom8030043
Ruhe J, Waldeyer C, Ojeda F, Altay A, Schnabel RB, Schaefer S, Lackner KJ, Blankenberg S, Zeller T, Karakas M (2018) Intrinsic iron release is associated with lower mortality in patients with stable coronary artery disease: first report on the prospective relevance of intrinsic iron release. Biomolecules 8:72. https://doi.org/10.3390/biom8030072
Grammer TB, Scharnagl H, Dressel A, Kleber ME, Silbernagel G, Pilz S, Tomaschitz A, Koenig W, Mueller-Myhsok B, März W, Strnad P (2019) Iron metabolism, hepcidin, and mortality (the Ludwigshafen Risk and Cardiovascular Health Study). Clin Chem 65:849–861. https://doi.org/10.1373/clinchem.2018.297242
Leaf DE, Rajapurkar M, Lele SS, Mukhopadhyay B, Boerger EAS, Mc Causland FR, Eisenga MF, Singh K, Babitt JL, Kellum JA, Palevsky PM, Christov M, Waikar SS (2019) Iron, hepcidin, and death in human AKI. J Am Soc Nephrol 30:493–504. https://doi.org/10.1681/ASN.2018100979
Klip IT, Voors AA, Swinkels DW, Bakker SJ, Kootstra-Ros JE, Lam CS, van der Harst P, van Veldhuisen DJ, van der Meer P (2017) Serum ferritin and risk for new-onset heart failure and cardiovascular events in the community. Eur J Heart Fail 19:348–356. https://doi.org/10.1002/ejhf.622
Vela D (2019) Systemic and local hepcidin as emerging and important peptides in renal homeostasis and pathology. BioFactors 45:118–134. https://doi.org/10.1002/biof.1468
Min HK, Oh YK, Choi KH, Lee KB, Park SK, Ahn C, Lee SW (2019) Relationship between cardiac geometry and serum hepcidin in chronic kidney disease: analysis from the KNOW-CKD Study. J Korean Med Sci 35:e2. https://doi.org/10.3346/jkms.2020.35.e2
Ulu SM, Yuksel S, Altuntaş A, Kacar E, Ahsen A, Altug A, Celik S, Sezer MT (2014) Associations between serum hepcidin level, FGF-21 level and oxidative stress with arterial stiffness in CAPD patients. Int Urol Nephrol 46:2409–2414. https://doi.org/10.1007/s11255-014-0753-7
Zhang X, Jin M, Wu H, Nadasdy T, Nadasdy G, Harris N, Green-Church K, Nagaraja H, Birmingham DJ, Yu CY, Hebert LA, Rovin BH (2008) Biomarkers of lupus nephritis determined by serial urine proteomics. Kidney Int 74:799–807. https://doi.org/10.1038/ki.2008.316
Zhang X, Nagaraja HN, Nadasdy T, Song H, McKinley A, Prosek J, Kamadana S, Rovin BH (2012) A composite urine biomarker reflects interstitial inflammation in lupus nephritis kidney biopsies. Kidney Int 81:401–406. https://doi.org/10.1038/ki.2011.354
Fufaa GD, Weil EJ, Nelson RG, Hanson RL, Knowler WC, Rovin BH, Wu H, Klein JB, Mifflin TE, Feldman HI, Vasan RS, Kimmel PL, Kusek JW, Mauer M, CKD Biomarkers Consortium and the RASS Investigators (2015) Urinary monocyte chemoattractant protein-1 and hepcidin and early diabetic nephropathy lesions in type 1 diabetes mellitus. Nephrol Dial Transplant 30:599–606. https://doi.org/10.1093/ndt/gfv012
Hayder ZS, Kareem ZS (2020) Resistin hormone in diabetic kidney disease and its relation to iron status and hepcidin. Int Urol Nephrol 52:749–756. https://doi.org/10.1007/s11255-020-02434-w
Prowle JR, Ostland V, Calzavacca P, Licari E, Ligabo EV, Echeverri JE, Bagshaw SM, Haase-Fielitz A, Haase M, Westerman M, Bellomo R (2012) Greater increase in urinary hepcidin predicts protection from acute kidney injury after cardiopulmonary bypass. Nephrol Dial Transplant 27:595–602. https://doi.org/10.1093/ndt/gfr387
Wagner M, Ashby DR, Kurtz C, Alam A, Busbridge M, Raff U, Zimmermann J, Heuschmann PU, Wanner C, Schramm L (2015) Hepcidin-25 in diabetic chronic kidney disease is predictive for mortality and progression to end stage renal disease. PLoS ONE 10:e0123072. https://doi.org/10.1371/journal.pone.0123072
Nakanishi T, Kuragano T, Nanami M, Nagasawa Y, Hasuike Y (2019) Misdistribution of iron and oxidative stress in chronic kidney disease. Free Radic Biol Med 133:248–253. https://doi.org/10.1016/j.freeradbiomed.2018.06.025
Hsieh YP, Huang CH, Lee CY, Chen HL, Lin CY, Chang CC (2013) Hepcidin-25 negatively predicts left ventricular mass index in chronic kidney disease patients. World J Nephrol 2:38–43. https://doi.org/10.5527/wjn.v2.i2.38
Kuragano T, Itoh K, Shimonaka Y, Kida A, Furuta M, Kitamura R, Yahiro M, Nanami M, Otaki Y, Hasuike Y, Nonoguchi H, Nakanishi T (2011) Hepcidin as well as TNF-α are significant predictors of arterial stiffness in patients on maintenance hemodialysis. Nephrol Dial Transplant 26:2663–2667. https://doi.org/10.1093/ndt/gfq760
Cano-Megías M, Guisado-Vasco P, Bouarich H, de la Fuente GA, de Sequera-Ortiz P, Álvarez-Sanz C, Rodríguez-Puyol D (2019) Coronary calcification as a predictor of cardiovascular mortality in advanced chronic kidney disease: a prospective long-term follow-up study. BMC Nephrol 20:188. https://doi.org/10.1186/s12882-019-1367-1
Abdel-Khalek MA, El-Barbary AM, Essa SA-M, Ghobashi AS (2011) Serum hepcidin: a direct link between anemia of inflammation and coronary artery atherosclerosis in patients with rheumatoid arthritis. J Rheumatol 38:2153–2159. https://doi.org/10.3899/jrheum.110339
Spoto B, Kakkar R, Lo L, Devalaraja M, Pizzini P, Torino C, Leonardis D, Cutrupi S, Tripepi G, Mallamaci F, Zoccali C (2019) Serum erythroferrone levels associate with mortality and cardiovascular events in hemodialysis and in CKD patients: a two cohorts study. J Clin Med 8:523. https://doi.org/10.3390/jcm8040523
Jankowska EA, Drozd M, Ponikowski P (2017) Iron deficiency treatment in patients with heart failure. Handb Exp Pharmacol 243:561–576. https://doi.org/10.1007/164_2017_30
Florian A, Ludwig A, Rösch S, Yildiz H, Klumpp S, Sechtem U, Yilmaz A (2014) Positive effect of intravenous iron-oxide administration on left ventricular remodelling in patients with acute ST-elevation myocardial infarction–A cardiovascular magnetic resonance (CMR) study. Int J Cardiol 173:184–189. https://doi.org/10.1016/j.ijcard.2014.02.016
Dziegala M, Kasztura M, Kobak K, Bania J, Banasiak W, Ponikowski P, Jankowska EA (2016) Influence of the availability of iron during hypoxia on the genes associated with apoptotic activity and local iron metabolism in rat H9C2 cardiomyocytes and L6G8C5 skeletal myocytes. Mol Med Rep 14:3969–3977. https://doi.org/10.3892/mmr.2016.5705
Haddad S, Wang Y, Galy B, Korf-Klingebiel M, Hirsch V, Baru AM, Rostami F, Reboll MR, Heineke J, Flögel U, Groos S, Renner A, Toischer K, Zimmermann F, Engeli S, Jordan J, Bauersachs J, Hentze MW, Wollert KC, Kempf T (2017) Iron-regulatory proteins secure iron availability in cardiomyocytes to prevent heart failure. Eur Heart J 38:362–372. https://doi.org/10.1093/eurheartj/ehw333
Li JJ, Meng X, Si HP, Zhang C, Lv HX, Zhao YX, Yang JM, Dong M, Zhang K, Liu SX, Zhao XQ, Gao F, Liu XL, Cui TX, Zhang Y (2012) Hepcidin destabilizes atherosclerotic plaque via overactivating macrophages after erythrophagocytosis. Arterioscler Thromb Vasc Biol 32:1158–1166. https://doi.org/10.1161/ATVBAHA.112.246108
Valenti L, Dongiovanni P, Motta BM, Swinkels DW, Bonara P, Rametta R, Burdick L, Frugoni C, Fracanzani AL, Fargion S (2011) Serum hepcidin and macrophage iron correlate with MCP-1 release and vascular damage in patients with metabolic syndrome alterations. Arterioscler Thromb Vasc Biol 31:683–690. https://doi.org/10.1161/ATVBAHA.110.214858
Saeed O, Otsuka F, Polavarapu R, Karmali V, Weiss D, Davis T, Rostad B, Pachura K, Adams L, Elliott J, Taylor WR, Narula J, Kolodgie F, Virmani R, Hong CC, Finn AV (2012) Pharmacological suppression of hepcidin increases macrophage cholesterol efflux and reduces foam cell formation and atherosclerosis. Arterioscler Thromb Vasc Biol 32:299–307. https://doi.org/10.1161/ATVBAHA.111.240101
Geng H, Wang A, Rong G, Zhu B, Deng Y, Chen J, Zhong R (2010) The effects of ox-LDL in human atherosclerosis may be mediated in part via the toll-like receptor 4 pathway. Mol Cell Biochem 342:201–206. https://doi.org/10.1007/s11010-010-0484-8
Xu XH, Shah PK, Faure E, Equils O, Thomas L, Fishbein MC, Luthringer D, Xu XP, Rajavashisth TB, Yano J, Kaul S, Arditi M (2001) Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation 104:3103–3108. https://doi.org/10.1161/hc5001.100631
Theurl I, Theurl M, Seifert M, Mair S, Nairz M, Rumpold H, Zoller H, Bellmann-Weiler R, Niederegger H, Talasz H, Weiss G (2008) Autocrine formation of hepcidin induces iron retention in human monocytes. Blood 111:2392–2399. https://doi.org/10.1182/blood-2007-05-090019
Peyssonnaux C, Zinkernagel AS, Datta V, Lauth X, Johnson RS, Nizet V (2006) TLR4-dependent hepcidin expression by myeloid cells in response to bacterial pathogens. Blood 107:3727–3732. https://doi.org/10.1182/blood-2005-06-2259
Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB, Liao D, Nagy L, Edwards PA, Curtiss LK, Evans RM, Tontonoz P (2001) A PPARγ-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell 7:161–171. https://doi.org/10.1016/s1097-2765(01)00164-2
Malhotra R, Wunderer F, Barnes HJ, Bagchi A, Buswell MD, O’Rourke CD, Slocum CL, Ledsky CD, Peneyra KM, Sigurslid H, Corman B, Johansson KB, Rhee DK, Bloch KD, Bloch DB (2019) Hepcidin deficiency protects against atherosclerosis. Arterioscler Thromb Vasc Biol 39:178–187. https://doi.org/10.1161/ATVBAHA.118.312215
Nairz M, Theurl I, Wolf D, Weiss G (2016) Iron deficiency or anemia of inflammation? Differential diagnosis and mechanisms of anemia of inflammation. Wien Med Wochenschr 166:411–423. https://doi.org/10.1007/s10354-016-0505-7
Girelli D, Nemeth E, Swinkels DW (2016) Hepcidin in the diagnosis of iron disorders. Blood 127:2809–2813. https://doi.org/10.1182/blood-2015-12-639112
Camaschella C, Nai A, Silvestri L (2020) Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica 105:260–272. https://doi.org/10.3324/haematol.2019.232124
Larson DS, Coyne DW (2013) Understanding and exploiting hepcidin as an indicator of anemia due to chronic kidney disease. Kidney Res Clin Pract 32:11–15. https://doi.org/10.1016/j.krcp.2013.01.001
Galesloot TE, Vermeulen SH, Geurts-Moespot AJ, Klaver SM, Kroot JJ, van Tienoven D, Wetzels JF, Kiemeney LA, Sweep FC, den Heijer M, Swinkels DW (2011) Serum hepcidin: reference ranges and biochemical correlates in the general population. Blood 117:e218–e225. https://doi.org/10.1182/blood-2011-02-337907
Macdougall IC, Bircher AJ, Eckardt KU, Obrador GT, Pollock CA, Stenvinkel P, Swinkels DW, Wanner C, Weiss G, Chertow GM, Participants C (2016) Iron management in chronic kidney disease: conclusions from a “Kidney disease: improving global outcomes”(KDIGO) controversies conference. Kidney Int 89:28–39. https://doi.org/10.1016/j.kint.2015.10.002
Acknowledgments
None to declare
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
REA is the co-owner of the project, wrote the manuscript, and approved the version to be published. MK reviewed the literature, helped in writing the manuscript, and approved the version to be published. AI helped in writing the manuscript and approved the version to be published. BA is the co-owner of the project, revised it critically for important intellectual content, and approved the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
None to declare.
Informed consent
Consent for publication: On behalf of all the authors, the corresponding Author declares that this manuscript is original, has not been published before, and is not currently being considered for publication elsewhere. He declares that the manuscript has been read and approved by all named authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Afsar, R.E., Kanbay, M., Ibis, A. et al. In-depth review: is hepcidin a marker for the heart and the kidney?. Mol Cell Biochem 476, 3365–3381 (2021). https://doi.org/10.1007/s11010-021-04168-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-021-04168-4